Bubonic Plague, Smallpox, and Tuberculosis (TB): Epidemics and Diseases
The Bubonic Plague, Smallpox, and Tuberculosis (TB) are three of the most historically significant and devastating diseases that have impacted humanity. Each has had a profound effect on societies, populations, and public health practices.
Here’s a summary of each in the context of epidemics and diseases:
Bubonic Plague (The Black Death)
- Pathogen: Caused by the bacterium Yersinia pestis.
- Transmission: Primarily transmitted to humans through the bite of infected fleas, which are often carried by rodents like rats. It can also spread through direct contact with infected animal tissue or, in its pneumonic form, via airborne droplets from person to person.
- Epidemics: The most infamous outbreak was the Black Death (1347-1351), which wiped out an estimated one to two-thirds of Europe’s population (over 25 million people). This was the first of three major plague pandemics that spanned from the 1300s to the 1900s, collectively killing millions of people. Later outbreaks include the Great Plague of London (1665-1666) and the third pandemic, which began in China in 1855 and spread globally, causing an estimated 12 million deaths in India alone between 1898 and 1918.
- Characteristics: Symptoms typically include fever, headache, chills, and weakness, along with characteristic swollen, painful lymph nodes called “buboes” (hence “bubonic”). Without treatment, it can quickly progress to septicemic (blood infection) or pneumonic (lung infection) plague, both of which are highly lethal.
- Modern Status: While dramatically reduced, plague still exists today, with isolated outbreaks occurring in various parts of the world, including Madagascar, the Democratic Republic of Congo, and the western United States. It is treatable with antibiotics if diagnosed early.
Smallpox
- Pathogen: Caused by the variola virus.
- Transmission: Spread primarily through direct and prolonged face-to-face contact with an infected person. It could also spread through contaminated objects like bedding or clothing.
- Epidemics: Smallpox has afflicted humanity for millennia, with evidence found in ancient Egyptian mummies. It repeatedly caused devastating epidemics across continents. Its introduction to the Americas by European explorers in the 16th century decimated indigenous populations, contributing to the collapse of empires like the Aztecs and Inca. Historically, it killed millions annually and was a major cause of blindness and severe scarring.
- Characteristics: Symptoms included fever, body aches, and a distinctive rash that progressed to fluid-filled pustules, which then scabbed over. The fatality rate was high, often around 30%.
- Modern Status: Smallpox holds a unique place in history as the first (and to date, only) human infectious disease to be globally eradicated. This monumental achievement was the result of a concerted worldwide vaccination campaign led by the World Health Organization, culminating in its official declaration of eradication in 1980. There are no naturally occurring cases today.
Tuberculosis (TB)
- Pathogen: Caused by the bacterium Mycobacterium tuberculosis.
- Transmission: Primarily airborne. It spreads when an infected person with active pulmonary TB coughs, sneezes, or speaks, expelling bacteria into the air that others can then inhale.
- Epidemics: Historically known as “consumption” or the “white plague,” TB has been a persistent and widespread killer for centuries. It ravaged populations in crowded urban areas, particularly during the Industrial Revolution. Its impact was so profound that it was once responsible for a quarter of all adult deaths in Europe and the Americas in the 18th and 19th centuries.
- Characteristics: Most commonly affects the lungs, causing a persistent cough, chest pain, coughing up blood or phlegm, fatigue, weight loss, fever, and night sweats. It can also affect other parts of the body. Many people carry the latent form of the bacteria without symptoms, but it can become active if their immune system is weakened.
- Modern Status: Despite being preventable and curable, TB remains one of the deadliest infectious diseases globally, especially in developing countries. Over a million people still die from TB each year. The rise of drug-resistant strains (MDR-TB and XDR-TB) poses a significant public health challenge, making treatment more complex and lengthy. Global efforts focus on early diagnosis, effective treatment, and public health surveillance.
These diseases underscore the complex interplay between pathogens, human populations, and environmental factors, as well as the ongoing challenge of public health in preventing and controlling infectious diseases.
Bubonic Plague (The Black Death)

Citizens of Tournai bury plague victims. Miniature from The Chronicles of Gilles Li Muisis (1272–1352). Bibliothèque royale de Belgique, MS 13076–77, f. 24v.
(Wiki image By Pierart dou Tielt (fl. 1340-1360) – http://balat.kikirpa.be/photo.php?path=X004175&objnr=20049662, Public Domain, https://commons.wikimedia.org/w/index.php?curid=64384803)
Bubonic Plague Quotes
The Bubonic Plague, particularly during the devastating Black Death of the 14th century, evoked immense fear, despair, and profound observations on human nature, society, and mortality.
Here are some quotes related to the Bubonic Plague, reflecting the historical accounts and philosophical interpretations:
From Historical Accounts & Contemporary Observers of the Black Death:
- “Neither physicians nor medicines were effective. Whether because these illnesses were previously unknown or because physicians had not previously studied them, there seemed to be no cure. There was such a fear that no one seemed to know what to do. When it took hold in a house, it often happened that no one remained who had not died.” — Marchione di Coppo Stefani (Florentine chronicler)
- “How many valiant men, how many fair ladies, breakfast with their kinfolk and the same night supped with their ancestors in the next world! The condition of the people was pitiable to behold. They sickened by the thousands daily, and died unattended and without help. Many died in the open street, others died in their houses, made it known by the stench of their rotting bodies.” — Giovanni Boccaccio (From The Decameron, set during the Black Death)
- “Realizing what a deadly disaster had come to them, the people quickly drove the Italians from their city. However, the disease remained, and soon death was everywhere. Fathers abandoned their sick sons. Lawyers refused to come and make out wills for the dying. Friars and nuns were left to care for the sick, and monasteries and convents were soon deserted, as they were stricken, too. Bodies were left in empty houses, and there was no one to give them a Christian burial.” — Unknown chronicler.
- “It struck me very deep this afternoon going with a hackney coach from my Lord Treasurer’s down Holborne, the coachman I found to drive easily and easily, at last stood still, and came down hardly able to stand, and told me that he was suddenly struck very sick, and almost blind, he could not see. So I ‘light and went into another coach with a sad heart for the poor man and trouble for myself lest he should have been struck with the plague, being at the end of town that I took him up; But God have mercy upon us all!” — Samuel Pepys (From his Diary, during the Great Plague of London, 1665)
From Literary & Philosophical Works (often reflecting on the nature of plague):
- “He knew what those jubilant crowds did not know but could have learned from books: that the plague bacillus never dies or disappears for good; that it can lie dormant for years and years in furniture and linen chests; that it bides its time in bedrooms, cellars, trunks, and bookshelves; and that perhaps the day would come when, for the bane and the enlightening of men, it would rouse up its rats again and send them forth to die in a happy city.” — Albert Camus (From The Plague, a fictional work that uses a plague outbreak as an allegory for human existence and resistance)
- “Thus the first thing that plague brought to our town was exile.” — Albert Camus (From The Plague)
- “What we learn in time of pestilence: that there are more things to admire in men than to despise.” — Albert Camus (From The Plague)
These quotes capture the terror, the societal breakdown, the human resilience, and the philosophical contemplation sparked by such a devastating disease.
Bubonic Plague YouTube Video
- Plague 101 | National Geographic – 2,356,200 views
- Why the Bubonic Plague Still Exists Today | Seeker – 221,941 views
- What Made The Black Death (The Plague) so Deadly? | The Infographics Show – 8,605,763 views
- Why plague doctors wore beaked masks | TED-Ed – 1,933,889 views
- Why bubonic plague lingers in US: ‘Black Death’ symptoms and treatment. #Shorts | USA TODAY – 24,840 views
Bubonic Plague Books
The Bubonic Plague, specifically the pandemic known as the Black Death (1347–1351), has been the subject of numerous historical, academic, and fictional books.
Here is a selection of highly regarded and popular books, categorized by genre:
Non-Fiction: Historical Accounts
These books provide a comprehensive historical analysis of the Black Death, its causes, spread, and social impact.
-
A Distant Mirror: The Calamitous 14th Century by Barbara W. Tuchman 📜
-
While not solely about the plague, the Black Death serves as the central catastrophic event in this acclaimed narrative history of 14th-century Europe. Tuchman uses vivid detail to show how the plague fractured medieval society.
-
-
The Great Mortality: An Intimate History of the Black Death, the Most Devastating Plague of All Time by John Kelly 🔬
-
This is a detailed, narrative history that traces the plague’s path from Central Asia to Europe, blending scientific research with medieval accounts to provide a visceral and comprehensive portrait of the pandemic.
-
-
The Black Death: A Personal History by John Hatcher 👤
-
A unique blend of history and historical fiction, Hatcher draws on his extensive knowledge of the period to create a deeply personal, intimate narrative of the plague’s arrival and its effects on English villages.
-
-
The Black Death by Philip Ziegler 🏰
-
First published in 1969, this remains a classic, foundational work on the subject, offering a scholarly yet accessible overview of the plague’s history and societal consequences.
-
Classic and Literary Fiction
These foundational works are set during or are heavily framed by the plague’s devastating presence.
-
The Decameron by Giovanni Boccaccio 🍷
-
Written shortly after the Black Death in Florence (c. 1353), this classic collection of 100 tales is framed by a group of seven young women and three young men who flee the plague-ridden city to a secluded villa where they amuse themselves by telling stories.
-
-
A Journal of the Plague Year by Daniel Defoe ✍️
-
A fictional memoir, published in 1722, that recounts the experiences of a man living in London during the Great Plague of 1665 (a later, but related, outbreak of the bubonic plague), rendered with such realistic detail that it is often mistaken for a true historical diary.
-
-
The Plague by Albert Camus 🩺
-
Though set in the 1940s in the Algerian town of Oran, this classic existentialist novel uses the outbreak of a fictional plague as an allegory to explore themes of absurdity, human morality, and the struggle against fate.
-
Modern Historical Fiction
These popular novels use the plague as a setting or a major plot catalyst.
-
Year of Wonders by Geraldine Brooks 🐑
-
Based on the true story of the English village of Eyam in 1666, which chose to quarantine itself to prevent the spread of the plague. The novel follows a young woman as she navigates the aftermath of a death, faith, and superstition.
-
-
Doomsday Book by Connie Willis 🕰️
-
A science fiction novel involving time travel. A modern historian travels back to 14th-century Oxford, intending to study medieval life, only to become trapped in England just as the Black Death begins.
-
-
World Without End by Ken Follett 🔨
-
The second novel in the Kingsbridge series is an epic historical drama set in 14th-century England, where the characters must face the arrival of the Black Death, which completely reshapes their society and fortunes.
-
-
Hamnet by Maggie O’Farrell 🎭
-
This novel centers on the family of William Shakespeare in 1596 and the death of his son, Hamnet, from what is believed to be the bubonic plague, exploring the grief and creation that resulted from the tragedy.
-
Bubonic Plague Science

An Oriental rat flea (Xenopsylla cheopis) infected with the plague bacterium (Yersinia pestis) appears as a dark mass in the gut. A Y. pestis biofilm blocks the foregut of this flea; when the flea attempts to feed on an uninfected host, Y. pestis from the foregut is regurgitated into the wound, causing infection.
- Yersinia pestis bacteria:
- Bubonic Plague Diagnostic Methods:
- Bubonic Plague Microscopic Images:
(Wiki Image By National Institute of Allergies and Infectious Diseases-photographer not listed – http://www.niaid.nih.gov/labsandresources/labs/aboutlabs/lzp/plaguesection/Pages/hinnebusch.aspx, Public Domain, https://commons.wikimedia.org/w/index.php?curid=17550053)
Bubonic plague is an infectious disease caused by the bacterium Yersinia pestis. This zoonotic bacterium is typically found in small mammals and their fleas, which act as vectors for transmission. While it may seem like a disease of the past, bubonic plague still exists today in various parts of the world, though outbreaks are much less severe due to modern medicine.
Here’s a breakdown of the science behind bubonic plague:
- The Causative Agent: Yersinia pestis
- Yersinia pestis is a Gram-negative coccobacillus bacterium.
- It’s a highly virulent pathogen capable of causing severe disease in humans and animals.
- Genetic analysis of ancient Y. pestis strains from plague pits (mass graves) has shown that the Black Death strain is ancestral to all modern circulating strains, indicating that the medieval pandemic was a foundational event in the bacterium’s evolution.
- Transmission:
- Flea Bites: The most common mode of transmission to humans is through the bite of infected fleas. Fleas acquire the bacteria from infected rodents (like rats, mice, squirrels, etc.). When the infected rodent host dies, the fleas seek new hosts, including humans.
- Direct Contact: Unprotected contact with infectious bodily fluids or contaminated tissues from an infected animal or human can also lead to infection.
- Inhalation (Pneumonic Plague): While bubonic plague is rarely transmitted person-to-person, a more severe form called pneumonic plague (affecting the lungs) can spread through respiratory droplets from an infected individual.
- Pathogenesis (How the Disease Progresses):
- After an infected flea bite, Y. pestis enters the body and travels through the lymphatic system to the nearest lymph node.
- In the lymph node, the bacteria replicate rapidly, causing inflammation, tension, and severe pain. These swollen, painful lymph nodes are called “buboes,” giving the disease its name.
- At advanced stages, the buboes can become open sores filled with pus.
- Y. pestis can resist phagocytosis (engulfment by immune cells) and even reproduce inside immune cells, effectively disarming the body’s defenses.
- If left untreated, the infection can spread from the lymph nodes to the bloodstream (leading to septicemic plague) or to the lungs (leading to pneumonic plague), both of which are far more dangerous and often fatal.
- Symptoms:
- Symptoms typically appear after an incubation period of one to seven days and can include:
- Sudden onset of high fever and chills
- Headaches and body aches
- Weakness, nausea, and vomiting
- The characteristic painful, swollen lymph nodes (buboes)
- Diagnosis and Treatment:
- Diagnosis: Confirmation of plague requires laboratory testing, ideally by identifying Y. pestis from a sample of pus from a bubo, blood, or sputum.
- Treatment: Early diagnosis and prompt antibiotic treatment are crucial for survival. Antibiotics like streptomycin are highly effective, and recovery rates are high if treated within 24 hours of symptom onset, especially for pneumonic plague.
- Scientific Research and Impact:
- Genomic Studies: Modern genomic analyses of ancient Y. pestis DNA have provided critical insights into the evolution of the bacterium and the devastating impact of historical pandemics like the Black Death.
- Human Evolution: The Black Death had a profound impact on human genetics. Research has identified genetic variants that became more common in populations after the Black Death, suggesting natural selection for genes that provided some protection against the disease. Interestingly, some of these protective variants may also be associated with an increased risk of autoimmune diseases today.
- Ongoing Surveillance: Although rare in most developed countries, Yersinia pestis is still endemic in some parts of the world (e.g., Democratic Republic of the Congo, Madagascar, Peru). Scientific research continues to focus on understanding its molecular interactions, identifying ecological and social determinants of outbreaks, and improving diagnosis and treatment to prevent future widespread epidemics.
Understanding the scientific basis of bubonic plague is vital for controlling current outbreaks and for comprehending its historical impact on human populations and evolution.
Bubonic Plague Epidemics. Table
While I cannot create a real-time, dynamic table of current epidemics, I can provide a comprehensive table summarizing the major historical epidemics and outbreaks of bubonic plague. It’s essential to recall that the bubonic plague is a disease that has caused numerous epidemics throughout history.
Here is a table outlining some of the most significant bubonic plague epidemics:
Major Bubonic Plague Epidemics Throughout History
| Epidemic Name / Period | Time Period (Approximate) | Geographic Scope | Estimated Mortality (Range) | Key Characteristics & Impact |
| Plague of Justinian | 541 – 750 AD (recurrent outbreaks) | Byzantine Empire (Mediterranean Basin), Western Europe, Near East | 25-50 million (initial wave); potentially 100 million over 2 centuries | One of the earliest well-documented plague pandemics. Caused massive depopulation, weakened the Byzantine Empire, and contributed to the decline of urban centers. Considered a Yersinia pestis event. |
| The Black Death | 1346 – 1353 AD (initial wave) | Europe, Asia, North Africa | 75-200 million (initial wave); estimated 30-60% of Europe’s population | The most devastating pandemic in human history. Led to profound social, economic, political, and religious upheaval. Recurrent outbreaks continued for centuries after the initial wave. |
| Second Plague Pandemic (Post-Black Death Recurrences) | 1353 – early 19th Century | Europe, Asia, North Africa | Millions over centuries (fluctuating regionally) | This refers to the series of recurrent outbreaks that followed the initial Black Death. Cities like London (Great Plague of London, 1665-1666) and Marseille (Great Plague of Marseille, 1720-1722) experienced significant localized epidemics during this period. |
| Third Plague Pandemic | 1855 – 1959 AD (officially declared over) | Originating in Yunnan, China, it spread globally to port cities | ~12 million | Primarily spread by steamships and trade routes, reaching every inhabited continent. This led to the identification of Yersinia pestis and its transmission by fleas and rats. This pandemic still saw significant mortality but also the development of effective treatments. |
| Modern Sporadic Outbreaks | Late 20th Century – Present | Primarily in specific endemic regions (e.g., Madagascar, Democratic Republic of Congo, Peru, and parts of the USA) | Varies greatly; typically very low due to prompt treatment | Though not a “pandemic” in the historical sense, Yersinia pestis is still present. Outbreaks are usually localized and contained, with a much lower fatality rate due to modern antibiotics and public health measures. |
Export to Sheets
Important Considerations:
- Mortality Estimates: Figures for historical pandemics are estimates and can vary widely across sources due to limited record-keeping.
- Recurrence: Plague is a disease characterized by its recurring nature. Even after a major initial pandemic, localized outbreaks could re-emerge for centuries.
- Disease vs. Epidemic: Bubonic plague is the disease. An epidemic (or pandemic, which is a widespread epidemic) is the occurrence of a disease in a large number of people in a particular region or worldwide, exceeding what is normally expected.
- Modern Context: While the historical impact was catastrophic, modern science and medicine have significantly reduced the threat of plague epidemics. However, surveillance and preparedness are still crucial in endemic regions.
Bubonic Plague History

Copper engraving of a plague doctor from the 17th century. This is one of the most well-known representations in art of the bubonic plague.
(Wiki Image By I. Columbina, ad vivum delineavit. Paulus Fürst Excud〈i〉t. – Internet Archive’s copy of Eugen Holländer,Die Karikatur und Satire in der Medizin: Medico-Kunsthistorische Studie von Professor Dr. Eugen Holländer, 2nd edn (Stuttgart:Ferdinand Enke, 1921), fig. 79 (p. 171)., Public Domain, https://commons.wikimedia.org/w/index.php?curid=15677032)
- Bubonic Plague historical images:
- Black Death illustrations:
- Map of the spread of the Black Death in Europe:
The Bubonic Plague, caused by the bacterium Yersinia pestis, has a long and devastating history, characterized by multiple waves of pandemics that have profoundly reshaped human civilization. Its transmission primarily involves fleas living on rodents (especially rats), which then bite humans.
Historians and scientists generally identify three major plague pandemics:
- The First Pandemic: The Plague of Justinian (541–750 CE)
- Origin and Spread: This pandemic originated in East Africa (possibly Ethiopia or Egypt) and spread rapidly throughout the Byzantine Empire, the Mediterranean Basin, and parts of Europe, North Africa, and the Middle East, primarily via sea trade routes. The first major outbreak began in the port city of Pelusium, Egypt, in 541 CE, reaching Constantinople (the capital of the Byzantine Empire) by 542 CE.
- Impact: Named after the Byzantine Emperor Justinian I, this plague caused widespread death and economic disruption. It is estimated to have killed between 25 and 100 million people globally, severely weakening the Byzantine Empire and contributing to a decline in urban life and trade in the affected regions. Recurrent outbreaks continued intermittently for over two centuries.
- The Second Pandemic: The Black Death and its Recurrences (1331–Early 19th Century)
- Origin and Initial Spread (The Black Death): This is the most infamous and devastating pandemic. Genetic research suggests its origin lies in Central Asia, particularly in what is now Kyrgyzstan, with evidence of outbreaks in the 1330s. It spread westward along trade routes (like the Silk Road) and then via merchant ships.
- 1331-1347: Plague outbreaks were reported in China (where they caused massive death tolls in the 1330s and 1350s) and other parts of Asia.
- 1347: It reached Europe when Genoese merchant ships, fleeing the besieged Crimean port of Kaffa (where Mongols reportedly catapulted plague-infected corpses into the city), docked in Messina, Sicily.
- 1347-1351: The Black Death rapidly swept across Europe, North Africa, and the Middle East. It killed an estimated 30% to 60% of Europe’s population, roughly 25 to 50 million people, and around 33% of the Middle East’s population. Entire communities were annihilated.
- Recurrences (14th-18th Centuries): The Black Death was not a single event but the first and most severe wave of a pandemic that recurred every few generations for centuries. Notable later outbreaks include:
- Great Plague of London (1665-1666): Killed an estimated 100,000 people (about one-quarter of London’s population) just before the Great Fire of London, which ironically helped to reduce the rat population.
- Great Plague of Marseille (1720-1722): The last major outbreak of the plague in Western Europe.
- Moscow Plague of 1770-1771: A severe outbreak in Russia.
- Impact: The Black Death profoundly reshaped European society, resulting in severe labor shortages, social upheaval, economic changes (such as increased wages for survivors), and shifts in religious and cultural outlooks. It contributed to the decline of feudalism and spurred advancements in public health measures like quarantine (the word deriving from the Italian quarantena, meaning forty days of isolation for ships).
- The Third Pandemic (1855–1959)
- Origin and Spread: This pandemic began in Yunnan Province, China, around 1855. It spread rapidly, reaching Canton (Guangzhou) in 1894 and then Hong Kong. From there, it was carried by steamships, trade routes, and rat populations to ports worldwide, including India, Japan, Australia, North America, and South America.
- Impact: While global, this pandemic caused the most devastation in India, where an estimated 12 million people died between 1898 and 1918. It brought the plague to the attention of modern science.
- Scientific Breakthroughs: This period was crucial for understanding the disease. In 1894, during an outbreak in Hong Kong, Swiss-French bacteriologist Alexandre Yersin isolated the bacterium responsible, naming it Yersinia pestis (after himself). In 1898, Paul-Louis Simond discovered that fleas from infected rats were the primary vector for transmission, clarifying the disease’s epidemiology. These discoveries led to more effective control measures.
- Decline: The third pandemic gradually faded by the mid-20th century, largely due to improved sanitation, rodent control, and the development of antibiotics (though effective antibiotics were widely available much later than the peak of the pandemic).
Modern Status:
Today, isolated cases of bubonic plague still occur in various parts of the world, including parts of Africa (notably Madagascar and the Democratic Republic of Congo), Asia (e.g., China, Mongolia), and the western United States. While rare, it is treatable with antibiotics if diagnosed early. However, it remains a serious public health concern in endemic regions, and surveillance is critical to prevent new outbreaks.
The First Pandemic: The Plague of Justinian (541–750 CE): Origin and Spread
The Plague of Justinian (541–750 CE) marks the first well-documented pandemic of the bubonic plague caused by the bacterium Yersinia pestis. Its origin and spread were intrinsically linked to ancient trade routes and environmental factors.
Origin
- Central Asian Steppes (Likely Ultimate Origin): While there’s ongoing debate, scientific consensus, supported by genetic studies of Y. pestis strains, points to the Tian Shan mountain ranges in Central Asia (present-day Kyrgyzstan, Kazakhstan, and China) as the probable ultimate geographic origin of the strain responsible for the Plague of Justinian. This region is a natural reservoir for Y. pestis in wild rodent populations.
- Initial Outbreak Location (Proximate Origin): The first documented major outbreak in the Roman (Byzantine) Empire occurred in Pelusium, an Egyptian port city near the Suez Canal, in 541 CE. This port was a crucial hub for the grain trade, connecting the interior of Africa with the Mediterranean world.
Spread
The spread of the Plague of Justinian followed established trade networks facilitated by the movement of rats and their fleas, which carried the Yersinia pestis bacterium.
- From Origin to Initial Outbreak: The bacterium likely traveled from its Central Asian origins along overland and sea trade routes (possibly including the Silk Road) to the fertile Nile River valley in Egypt. Rodents and their infected fleas often stowed away on merchant ships and caravans, transported the disease.
- Mediterranean Basin Invasions (The First Wave, 541-544 CE):
- Egypt as a Hub: From Pelusium, the plague rapidly spread throughout Egypt (including Alexandria) and Palestine.
- To Constantinople: By 542 CE, the plague reached Constantinople, the capital of the Byzantine Empire. This was a critical point in its spread due to Constantinople’s immense population (hundreds of thousands) and its strategic location as a nexus of international trade (connecting the Black Sea, Aegean Sea, Middle East, and North Africa). Grain ships, essential for feeding the city, were a primary vector for infected rats and fleas. Procopius, a contemporary historian, recorded its devastating impact on the city.
- Across the Mediterranean: From Constantinople and other infected port cities, the plague spread rapidly across the entire Mediterranean Basin, affecting:
- North Africa (Libya, Carthage)
- Italian Peninsula (Rome, Ravenna)
- Sicily
- Parts of Gaul (modern France)
- The Near East (Syria, Cilicia, Mesopotamia, Persia)
- Recurrent Waves (541-750 CE): The Plague of Justinian was not a single event but a series of recurrent waves that washed over the Mediterranean world and surrounding regions for over two centuries. Historians have identified at least 18 major recurrences during this period.
- These later waves, though perhaps less impactful than the initial one, continued to suppress population growth, disrupt economies, and weaken empires, notably contributing to the decline of the Byzantine Empire and the rise of the Arab Caliphates.
- Archaeological evidence (ancient DNA analysis) has confirmed the spread of Y. pestis from victims at various sites across Germany, France, Spain, and the UK, demonstrating the pandemic’s extensive reach beyond areas documented solely in historical texts.
Key Factors in its Spread:
- Trade Routes: The primary driver. Ships carrying grain and other goods provided ideal transport for infected rodents and fleas.
- Urbanization: Dense, unhygienic urban centers with large populations and plentiful rats were perfect environments for the disease to take hold and spread.
- Military Campaigns: The movement of armies and their supply trains could also facilitate the spread of the plague by carrying infected animals.
- Climate Factors (Debated): Some theories suggest that climatic shifts (e.g., a “volcanic winter” around 536 CE leading to colder temperatures and famine) may have weakened populations, making them more susceptible, or altered rodent populations and their interactions with humans.
The Plague of Justinian was a devastating pandemic that significantly reshaped the late antique world, highlighting the interconnectedness of human populations through trade and the devastating power of zoonotic diseases when introduced into susceptible populations.
The First Pandemic: The Plague of Justinian (541–750 CE): Impact
The Plague of Justinian (541–750 CE) was a catastrophic pandemic that profoundly impacted the Byzantine Empire, the Mediterranean world, and beyond. Its effects were multifaceted, affecting demographics, the economy, politics, and society, although historians and scientists still debate the exact extent and long-term causal links.
- Demographic Impact
- Massive Mortality: This is the most direct and devastating impact. Estimates of the total death toll over two centuries of recurrent outbreaks vary widely, from 25 million to 100 million people, with some scholars suggesting it claimed 25-60% of the Mediterranean population in the initial wave. In Constantinople, at its peak, as many as 5,000 to 10,000 people per day were reportedly dying, potentially wiping out 40-50% of the city’s inhabitants.
- Population Decline: The recurrent waves of plague prevented sustained population growth for centuries. This had a long-term depressive effect on population levels across the affected regions.
- Labor Shortages: The high mortality, particularly among the working-age population (farmers, soldiers, artisans), led to severe labor shortages. This crippled agriculture, military recruitment, and urban services.
- Economic Impact
- Agricultural Decline: With a significant reduction in the rural labor force, vast tracts of farmland went untended. This led to a decline in agricultural output and food shortages, particularly in grain-dependent urban centers.
- Trade Disruption: Fear of contagion and a decline in available labor and goods disrupted local and long-distance trade routes. Port cities, which were centers of infection, saw their economic activity plummet.
- Tax Revenue Collapse: The widespread deaths and economic downturn meant a drastic reduction in tax revenues for the state. Emperor Justinian’s attempts to maintain tax collection, even from the deceased’s neighbors, further exacerbated the suffering and economic strain.
- Increased Wages (for survivors): In the aftermath of the plague, severe labor shortages led to a significant increase in the purchasing power of wages for surviving workers, as demand for labor far outstripped supply. This led to higher living standards for some laborers but came at the cost of immense population loss.
- Economic Stagnation: While some argue for stimulating specific sectors due to changing demand, the empire’s overall picture was one of prolonged economic strain and stagnation.
- Political and Military Impact
- Weakening of the Byzantine Empire: The plague struck the Byzantine Empire at a critical juncture during Emperor Justinian I’s ambitious campaigns to reconquer former Western Roman territories (the renovatio imperii).
- Military Manpower Loss: The death toll significantly depleted the imperial army and navy, making it difficult to sustain conquests and defend existing borders. This particularly affected the ongoing Gothic Wars in Italy.
- Financial Strain: The collapse in tax revenue hindered Justinian’s ability to fund his military campaigns and ambitious building projects (like the Hagia Sophia).
- Loss of Imperial Control: The weakened military and economic base made it harder for the Byzantine Empire to maintain control over its vast territories, contributing to the loss of newly reconquered lands and the rise of local powers.
- Impact on Justinian’s Reign: While Justinian himself survived the plague, his grand vision for reuniting the Roman Empire was severely hampered. The plague is often seen as a turning point that prevented the full realization of his imperial ambitions.
- Shift in Geopolitical Balance: The weakening of the Byzantine Empire may have indirectly contributed to the later success of the Arab Muslim conquests in the 7th century, as the recurrent plague waves severely weakened both the Byzantines and their Sasanian Persian rivals.
- Social and Cultural Impact
- Social Disruption: Mass deaths overwhelmed burial systems, leading to desperate measures like mass graves. Social order broke down in many affected areas, with reports of widespread despair, lawlessness, and a general sense of hopelessness.
- Psychological Trauma: Living through repeated waves of a deadly, mysterious disease undoubtedly inflicted profound psychological trauma on survivors, leading to increased fear, superstition, and possibly changes in religious practices (e.g., increased piety or, conversely, a questioning of faith).
- Changes in Urban Life: Cities became depopulated, and urban services deteriorated. Some argue it contributed to a shift away from large urban centers towards more rural, decentralized living in some regions.
- Artistic and Literary Reflections: Contemporary accounts, though sometimes exaggerated, provide a chilling glimpse into the horrors of the plague, influencing the period’s literature and historical narratives.
While recent scholarship has debated the maximalist view of the Plague of Justinian’s impact, particularly challenging the idea that it caused a universal and immediate societal collapse across the entire Mediterranean, there is still broad consensus that its localized effects were devastating and its recurrent nature for over two centuries undoubtedly contributed to significant demographic, economic, and political transformations in the Late Antique and Early Medieval periods. It marked a clear end to an era of Roman imperial ambitions and ushered in new historical dynamics.
The Second Pandemic: The Black Death and its Recurrences (1331–Early 19th Century): Origin and Initial Spread (The Black Death)
The Black Death, the initial and most devastating wave of the Second Plague Pandemic (1331–early 19th Century), was a pivotal event in human history. Its origin and rapid spread across continents were a testament to the interconnectedness of the medieval world, primarily through trade routes.
Origin
Recent scientific breakthroughs, particularly genetic analysis of Yersinia pestis strains from historical burial sites, have provided strong evidence for the Black Death’s origin:
- Central Asian Steppes (Specifically, Kyrgyzstan): Research published in 2022 identified a specific burial site in the Tian Shan mountains in modern-day Kyrgyzstan (near Lake Issyk-Kul) as the likely epicenter. Gravestones from the Kara-Djigach and Burana sites explicitly mention deaths from “pestilence” in 1338-1339 CE. Genetic sequencing of Y. pestis from these remains showed that this specific strain sits at the “Big Bang” of the plague’s genetic diversification, meaning it is ancestral to virtually all modern plague strains, including those of the Black Death in Europe.
- Rodent Reservoirs: This region is a known natural reservoir for Yersinia pestis in wild rodent populations, particularly marmots. Fluctuations in climate (such as a “Little Ice Age” starting around this time) may have disrupted these rodent populations, leading to an increased spillover of the bacteria to other animals and then to humans via infected fleas.
Initial Spread (The Black Death Wave, c. 1346-1353 CE)
From its likely Central Asian origin, the plague spread westward along well-established trade networks.
- Along the Silk Road and Overland Routes (c. 1330s-1340s):
- The plague likely traveled along the Silk Road, a vast network of overland trade routes connecting East Asia with the Middle East and Europe. Caravans, carrying goods, people, and, crucially, rats and their fleas, facilitated its slow but relentless westward movement.
- Early outbreaks, likely devastating, were reported in China in the 1330s, though specific details are scarce. It also spread through parts of India, Persia (Iran), Syria, and Egypt before reaching Europe.
- The disease reached the territories of the Golden Horde (part of the Mongol Empire) in Central Asia and Russia by the mid-1340s.
- The Siege of Kaffa (1346-1347 CE) – A Major Entry Point to Europe:
- The most famous account of the plague’s direct entry into the Mediterranean world involves the Genoese trading post of Kaffa (modern Feodosiya) on the Crimean Peninsula (Black Sea).
- In 1346, the city was besieged by the Mongol Golden Horde army under Jani Beg. The plague ravaged the Mongol army, and according to some historical accounts, they catapulted infected corpses into the besieged city. While the effectiveness of this “biological warfare” is debated, it’s clear that the plague entered Kaffa.
- Genoese merchants fleeing Kaffa by ship carried the plague with them across the Black Sea and into the Mediterranean.
- Mediterranean Ports (1347-1348 CE):
- Messina, Sicily (October 1347): One of the first European ports to be directly hit when “death ships” from the Black Sea docked there. The disease quickly spread inland.
- Constantinople (late 1347): A major trade hub, Constantinople also became infected, experiencing massive mortality.
- Other Italian Ports: From Sicily and other initial points, the plague rapidly spread to major Italian port cities such as Genoa and Venice (early 1348), then to Pisa, Florence, and the rest of the Italian peninsula.
- Marseille, France (January 1348): Another infected ship from the Black Sea arrived, quickly spreading the plague throughout the city and inland into France.
- North Africa: The plague simultaneously spread to cities such as Tunis and Alexandria via Mediterranean trade routes.
- Across Europe (1348-1351 CE):
- From the initial landing sites, the plague followed major land and river trade routes.
- France and Spain: Rapidly spread throughout France and into the Iberian Peninsula.
- England (June 1348): Arrived via ships from continental Europe (likely France or Italy) at ports like Melcombe Regis (Dorset) and Bristol, then quickly moved inland to London and the rest of England.
- Germany, Scandinavia, and Eastern Europe (1349-1351): The plague continued its march, affecting most of Western and Central Europe. Its spread in Eastern Europe was somewhat slower or less intense in some areas, potentially due to lower population density or less interconnected trade routes, but it still caused significant devastation.
The combination of a highly virulent pathogen, densely populated urban centers, and a vast network of interconnected trade routes (both overland and maritime) created the perfect storm for the Black Death to become the most catastrophic pandemic in recorded history.
The Second Pandemic: The Black Death and its Recurrences (1331–Early 19th Century): Recurrences (14th-18th Centuries)
The Black Death (1346-1353 CE) was just the opening act of the Second Plague Pandemic, a period of nearly 500 years during which Yersinia pestis repeatedly ravaged Europe, North Africa, and the Middle East. While the initial wave was the most devastating in terms of sheer mortality, the recurrent outbreaks prevented sustained demographic recovery and continued to shape societies.
Characteristics of the Recurrences:
- Frequency: After the Black Death, plague became endemic in many areas. Outbreaks occurred with remarkable frequency, often every 10-15 years in major cities, well into the 17th century. It was rare for a year between 1350 and 1700 to pass without a major plague outbreak somewhere in Europe.
- Varying Virulence and Mortality: While no single recurrence matched the universal devastation of the Black Death, some local outbreaks were still extraordinarily lethal. Mortality rates in later outbreaks often ranged from 10-30% of the affected population but could still be much higher in specific cities or regions (e.g., 50-60% in Naples, 1656-1657).
- Geographic Variation: The plague’s impact varied regionally. Some areas experienced more frequent or severe recurrences than others, often influenced by trade routes, population density, and local public health measures.
- Impact on Younger Generations: Later waves, such as the one in 1360-1362 (sometimes called the “Children’s Plague”), disproportionately affected children and young adults who had not been exposed to the Black Death and thus lacked immunity.
- Persistent Economic and Social Strain: The continuous threat of plague prevented sustained population growth, perpetuating labor shortages, disrupting economic activity, and creating a climate of fear and uncertainty for centuries. This led to ongoing social and economic transformations (e.g., shifts in land tenure, peasant revolts, changes in urban planning).
Notable Later Outbreaks (Examples):
The list of individual plague outbreaks during this period is immense, but some stand out due to their severity, historical documentation, or their role as some of the last major events of the pandemic in Western Europe:
- 1360-1362: The first major recurrence after the Black Death, hitting many regions of Europe again, with significant mortality, particularly among younger demographics.
- Italian Plagues (1575-1577, 1629-1631, 1656-1657): Italy experienced several devastating outbreaks.
- The Italian Plague of 1629-1631 (also known as the Great Plague of Milan) was particularly severe in northern Italy, claiming an estimated 1.7 million lives (around 35% of the population) and severely impacting the region.
- The Great Plague of Naples (1656-1657) wiped out a substantial portion of the population in southern Italy and Sicily.
- Great Plague of Seville (1647-1652): Severely impacted Spain, particularly its southern regions, with very high mortality.
- Great Plague of London (1665-1666):
- This was London’s last major bubonic plague epidemic, killing an estimated 100,000 people (about 20-25% of the city’s population).
- It led to widespread panic, the flight of the wealthy, and desperate public health measures like isolating infected households.
- While often mistakenly credited with ending the plague, the Great Fire of London (1666), which occurred shortly after the plague subsided, did not directly stop the epidemic but perhaps prevented its resurgence by destroying crowded, rat-infested housing.
- Great Plague of Vienna (1679): Caused significant mortality in the Habsburg capital.
- Great Plague of Marseille (1720-1722):
- This was the last major outbreak of bubonic plague in Western Europe.
- It arrived on a ship from the Levant, and, despite quarantine efforts, spread rapidly, killing an estimated 50,000 of Marseille’s 90,000 inhabitants, and another 50,000 in surrounding areas of Provence.
- This outbreak spurred more rigorous quarantine and public health measures in European ports.
- Russian Plague of 1770-1772: One of the last major outbreaks in Eastern Europe, particularly devastating Moscow, where over 100,000 people died.
Reasons for the Decline and Disappearance in Europe:
The reasons why the plague eventually disappeared from Europe in the early 19th century while remaining endemic in other parts of the world are complex and still debated:
- Improved Public Health Measures: Lessons learned from centuries of outbreaks led to more effective quarantines, lazarettos (quarantine hospitals), and sanitary practices in port cities.
- Changes in Rat Populations: A hypothesis suggests a shift in the dominant rat species from the black rat (Rattus rattus, more prone to living near humans and carrying infected fleas) to the brown rat (Rattus norvegicus, more resilient but less likely to live in close proximity to humans). This is debated, as evidence for this shift varies geographically.
- Increased Immunity in Human Populations: Repeated exposure may have led to some level of acquired immunity or a process of natural selection favoring individuals with genetic resistance (though this would be a very long-term effect).
- Evolution of Yersinia pestis: The bacterium itself may have evolved to become less virulent, though there’s little direct evidence for this.
- Better Housing and Sanitation: Over centuries, improved housing construction (less conducive to rat infestations) and overall better sanitation in some areas reduced the conditions necessary for large-scale outbreaks.
- Climate-driven Reintroductions: Newer research suggests that plague outbreaks in Europe were not necessarily sustained by permanent European rodent reservoirs but rather by repeated reintroductions of Y. pestis from Central Asian reservoirs via trade routes, possibly linked to climate fluctuations affecting rodent populations there. As these trade routes shifted or became less efficient at transporting infected animals, the reintroductions may have diminished.
The Second Plague Pandemic, with its relentless recurrences, fundamentally altered European demographics and socio-economic structures and profoundly influenced medieval and early modern thought and institutions.
The Second Pandemic: The Black Death and its Recurrences (1331–Early 19th Century): Impact
The Black Death (the initial wave of the Second Pandemic) and its subsequent recurrences for nearly 500 years (from 1331 to the early 19th century) had a monumental and transformative impact on Europe, North Africa, and the Middle East. Unlike the singular devastation of the Plague of Justinian, the recurrent nature of the Second Pandemic meant that societies faced a prolonged period of demographic instability and adaptation.
Here’s a breakdown of its key impacts:
- Demographic Catastrophe and Long-Term Depression
- Initial Devastation (Black Death): The first wave (1346-1353) alone is estimated to have killed 30% to 60% of Europe’s population, with some regions experiencing even higher mortality (e.g., up to two-thirds in parts of Italy). This translates to an estimated 75 to 200 million deaths globally.
- Recurrent Mortality: The subsequent waves, although individually less globally devastating than the Black Death, continued to cause significant mortality. Plague became endemic, flaring up every 10-15 years in many areas. This prevented sustained population recovery; Europe’s population did not regain its pre-1348 levels until the early to mid-16th century and in some areas, even later.
- Labor Shortages: The consistently high mortality led to chronic labor shortages across all sectors—agriculture, manufacturing, and services. This was a persistent problem that shaped economic and social developments for centuries.
- Economic Transformation
- End of Serfdom (Western Europe): The most profound economic impact was the collapse of the feudal system in much of Western Europe. With a drastically reduced workforce, surviving peasants and laborers gained unprecedented bargaining power. Landlords, desperate for labor, were forced to offer better wages, more favorable rents, and ultimately, commute labor services into money rents, leading to the decline of serfdom.
- Increased Wages and Standard of Living: Real wages for peasants and artisans increased significantly due to labor scarcity. This led to a higher standard of living for many survivors, who could afford better food, clothing, and even some luxury items previously out of reach.
- Agricultural Restructuring: Marginal lands were abandoned, and there was a shift in agriculture from labor-intensive grain cultivation to less labor-intensive pastoralism (raising livestock), which suited the smaller population and improved diets (more meat and dairy).
- Urbanization and Economic Specialization: While cities were often hit harder by the plague, they also recovered by attracting rural migrants. The increased wealth of some survivors and changing demand patterns stimulated urban industries and trade, leading to increased regional specialization.
- Impact on Wealth Distribution: Initially, the plague led to a decline in inequality as large patrimonies were fragmented by high mortality rates (due to partible inheritance systems). The rising wages for laborers also contributed to this reduction in wealth concentration, a trend that lasted until the 17th century in some areas.
- Social and Cultural Shifts
- Psychological Impact: The overwhelming presence of death led to widespread trauma, fatalism, and a preoccupation with mortality. This manifested in art (e.g., “Dance of Death” iconography), literature, and religious practices.
- Religious Responses:
- Increased Piety and Penance: Many sought solace and explanation in religion, leading to intense piety, flagellant movements (public self-flogging as penance), and charitable bequests to the Church.
- Loss of Faith/Challenge to Authority: Conversely, the failure of the Church to stop the plague and the high mortality among the clergy led some to question religious authority and practices. This erosion of trust may have contributed to the intellectual and religious ferment that eventually led to the Reformation.
- Persecution of Minorities: In times of panic and desperation, minorities, particularly Jews, were often scapegoated and accused of poisoning wells or spreading the disease. This led to horrific pogroms and massacres across Europe.
- Changes in Education and Language: The death of many scholars and clergy led to shifts in educational institutions. The need for practical knowledge in local languages sometimes spurred the use of vernacular languages in medical texts and other fields, broadening access to knowledge.
- Medical Advancements: The catastrophic failures of traditional medicine forced doctors to rethink their approaches. This experience, though painful, laid some of the groundwork for more empirical observation and the beginnings of public health measures like quarantine. Hospitals evolved from isolation centers to places of treatment.
- Political and Geopolitical Effects
- Weakening of Feudal Powers: The decline of serfdom and the shift in wealth to the peasantry undermined the traditional power base of the nobility, leading to class struggles and peasant revolts (e.g., the Peasants’ Revolt in England, the Jacquerie in France).
- Strengthening of Central Authority: In some regions, the weakening of local feudal lords allowed for the gradual strengthening of central monarchical power, as kings could assert more control over a fractured landscape.
- Military Changes: Labor shortages also impacted military recruitment, potentially leading to a greater reliance on mercenary armies or changes in military organization.
- Catalyst for Exploration: Some historians argue that the disruption of traditional overland trade routes to the East (due to plague and political instability) and the drive for new resources and markets contributed to the impetus for European maritime exploration, ultimately leading to the Age of Discovery.
In essence, the Black Death and its recurrences were not merely a series of biological events but a powerful engine of transformation that fundamentally reshaped medieval European society, politics, and economy, setting the stage for the early modern period and contributing to many of the defining characteristics of later European history.
Bubonic Plague The Third Pandemic (1855–1959): Origin and Spread
The Third Plague Pandemic, which officially lasted from 1855 to 1959 (though plague still exists today in endemic pockets), stands apart from the previous two pandemics due to its global reach facilitated by modern transportation and, critically, the scientific advancements that led to understanding its cause and transmission.
Origin
- Yunnan Province, Southwest China (c. 1855): The Third Pandemic is widely accepted to have originated in the remote Yunnan Province of southwest China around 1855. Plague had likely been endemic in this region for a longer period (some sources suggest outbreaks since the late 18th century), existing in natural reservoirs among wild rodents.
- Human Activity as a Catalyst: A significant factor in its emergence as a pandemic was the rapid influx of Han Chinese into Yunnan in the mid-19th century. This was driven by a boom in mining (particularly copper) and the opium trade, leading to increased human-animal contact and higher population densities. Increased transportation within the region brought people into contact with plague-infected fleas and rodents, facilitating spillover into human populations and then into growing urban areas.
Initial Spread from China
From Yunnan, the plague began its outward expansion:
- Across China: The disease spread within China along trade routes, including rivers (like the Red River and You Jiang), and through population movements, particularly those linked to conflict (such as the Muslim Rebellion) and the burgeoning opium trade.
- Guangzhou (Canton) and Hong Kong (1894): These major port cities became critical distribution centers. The plague reached Guangzhou (Canton) in 1894, resulting in significant mortality, and subsequently spread rapidly to Hong Kong in the same year. The crowded and unsanitary conditions in Hong Kong’s Sheung Wan district, combined with its high population density, allowed the disease to flourish.
Global Spread (Primarily via Maritime Trade)
The defining characteristic of the Third Pandemic’s global reach was the advent of steamships and global maritime trade. Infected rats and their fleas, often hidden in cargo, were transported across oceans to ports worldwide.
- To India (1896 onwards): India, particularly British Raj India, bore the brunt of the Third Pandemic’s mortality. The plague arrived in Bombay (Mumbai) in 1896, likely via ships from Hong Kong or other Chinese ports, and then spread rapidly throughout the subcontinent. India alone suffered an estimated 10 million deaths from plague between 1896 and 1918. Calcutta also experienced major outbreaks.
- To Southeast Asia: From Hong Kong, the plague spread to other Asian ports like Singapore, Taiwan (1896), and Japan.
- To Africa: Ports in Africa, such as Madagascar (1898) and Cape Town (1900), became infected through ship-borne transmission. Madagascar remains an endemic hotspot for plague today.
- To the Americas:
- Hawaii (1899): The plague reached Hawaii, leading to outbreaks and dramatic public health responses, including the infamous Honolulu Chinatown fire.
- Continental US (1900): The disease arrived in San Francisco’s Chinatown in 1900, initiating a series of outbreaks along the West Coast and eventually establishing sylvatic (wild rodent) plague reservoirs in the western United States, where it still exists today.
- South America: Ports like Buenos Aires (1900) and Rio de Janeiro (1908) also saw outbreaks.
- To Europe (Sporadic Introductions): While Europe had largely moved beyond the devastating recurrences of the Second Pandemic, the Third Pandemic still brought repeated introductions of plague into European ports (e.g., London 1896, Oporto 1899). However, due to improved sanitation, quarantine measures, and stronger public health infrastructure, these European outbreaks were generally smaller and more contained than those in Asia.
- Australia: Sydney experienced outbreaks in 1900.
Key Factors Facilitating Global Spread:
- Steamships: Dramatically reduced travel times, allowing infected rats and fleas to survive voyages and disembark at distant ports.
- Globalized Trade Networks: The expansion of international trade provided the pathways for the infected vectors and hosts.
- Urbanization and Poor Sanitation: Densely populated port cities with inadequate sanitation, high rat populations, and close human-rat contact were highly vulnerable to new introductions and rapid spread.
The Third Pandemic, while causing immense suffering, particularly in India and China, also played a crucial role in the scientific understanding of plague. It was during this pandemic that Alexandre Yersin (1894) and Shibasaburo Kitasato (1894) independently identified Yersinia pestis as the causative bacterium, and later, Paul-Louis Simond (1898) conclusively demonstrated the role of fleas as vectors, paving the way for more effective control measures and leading to its eventual decline as a global pandemic.
Bubonic Plague The Third Pandemic (1855–1959): Impact
The Third Plague Pandemic (1855–1959) had a profound and lasting impact, distinct from its predecessors primarily due to its overlap with the burgeoning fields of modern medicine, bacteriology, and global public health. While it didn’t cause the same level of societal upheaval as the Black Death, its consequences were significant, particularly in Asia.
- Massive Mortality (Especially in Asia)
- Global Death Toll: The Third Pandemic caused an estimated 12 to 15 million deaths worldwide.
- India’s Burden: British Raj India bore the overwhelming brunt of the pandemic, with an estimated 10 million deaths from plague between 1896 and 1918 alone. This made it one of the deadliest pandemics in India’s history.
- China’s Toll: China also experienced millions of deaths, particularly in the initial outbreaks in Yunnan, Guangzhou, and Hong Kong.
- Localized but Severe Outbreaks: While total European deaths were comparatively low (around 1,700 cases and 450 deaths between 1899-1947), outbreaks in port cities worldwide were often intense and deadly, leading to significant local mortality and disruption.
- Catalyst for Scientific Discovery
This is arguably the most significant long-term impact of the Third Pandemic. The presence of plague in rapidly globalizing port cities provided an urgent impetus for scientific investigation:
- Identification of Yersinia pestis: In 1894, during the Hong Kong outbreak, Alexandre Yersin (a Swiss-French bacteriologist) and Shibasaburo Kitasato (a Japanese bacteriologist) independently isolated the causative bacterium, which was later named Yersinia pestis in honor of Yersin. This discovery was a cornerstone of modern bacteriology.
- Identification of Flea Vector: In 1898, Paul-Louis Simond (another French bacteriologist) conclusively demonstrated the role of the rat flea (Xenopsylla cheopis) as the primary vector for transmission of plague from rats to humans. This was a crucial breakthrough in understanding the disease’s epidemiology.
- Development of Vaccines and Treatments:
- Early Vaccines: Scientists like Waldemar Haffkine developed early, though imperfect, plague vaccines (e.g., the inactivated bacterial vaccine in 1897) during the Indian outbreaks. These were initially tested on humans and contributed to some protection.
- Antibiotic Era: While the pandemic officially ended in 1959, the later development of antibiotics (like streptomycin in the 1940s) proved to be highly effective against Yersinia pestis. This transformed plague from a rapidly fatal disease into a curable one, significantly reducing mortality in affected regions by the mid-20th century.
- Transformation of Public Health Practices
The Third Pandemic forced governments and international bodies to develop more sophisticated and coordinated public health responses:
- Quarantine Reinforcement: The pandemic led to stricter and more systematic quarantine measures in ports globally, often involving the fumigation of ships to kill rats and fleas.
- Sanitation and Rat Control: Understanding the role of rats and fleas spurred widespread campaigns for improved urban sanitation, including better waste management, rat extermination efforts, and pest control (e.g., use of insecticides like DDT in later stages).
- International Cooperation: The global spread of the plague highlighted the need for international collaboration in disease surveillance and control. This laid some groundwork for later global health initiatives.
- Biomedical Research Focus: The successful identification of the pathogen and vector solidified the “germ theory of disease” and propelled investment in biomedical research.
- Social and Political Consequences
- Exacerbation of Social Inequalities: In many colonial contexts (e.g., British India, Hong Kong), the plague outbreaks highlighted and often exacerbated existing social and racial inequalities. European authorities frequently imposed harsh and culturally insensitive public health measures (like forced evacuations, segregation, and house searches) on local populations, leading to resentment and resistance.
- Economic Disruption: While not as globally catastrophic as the Black Death, localized outbreaks caused significant economic disruption in affected port cities, leading to trade halts, labor shortages, and financial losses.
- Migration and Urban Exodus: Fear of the plague often led to temporary or permanent exodus from affected cities, disrupting social structures and labor pools. The Honolulu Chinatown fire (1900), set by health officials attempting to control the plague, devastated a community.
- Political Instability (Localized): In some regions, severe outbreaks contributed to local political instability or fueled anti-colonial sentiments.
- Establishment of Endemic Foci
- Unlike the Second Pandemic, which largely disappeared from Europe, the Third Pandemic led to the establishment of new, permanent animal reservoirs (sylvatic plague) in various parts of the world where it had not been previously (e.g., the western United States, parts of Africa, South America). This means that Yersinia pestis continues to circulate in wild rodent populations in these areas, posing a sporadic risk to humans even today.
In summary, the Third Plague Pandemic was a period of immense human suffering, but it also became a crucible for modern microbiology and public health. Its legacy is not only a death toll but also the scientific understanding and public health infrastructure that ultimately brought plague under control, preventing it from having the same devastating impact in the 20th century as it had in previous eras.
Bubonic Plague The Third Pandemic (1855–1959): Decline
The Third Plague Pandemic, while officially declared over in 1959, did not end abruptly but rather saw a gradual and significant decline in its global impact, particularly in terms of human mortality. This decline was largely due to a confluence of scientific breakthroughs and evolving public health strategies.
Here are the key factors contributing to the decline of the Third Pandemic:
- Scientific Understanding of Plague (Late 19th Century)
- Discovery of the Pathogen (Yersinia pestis – 1894): The independent identification of the bacterium by Alexandre Yersin and Shibasaburo Kitasato in 1894 was a game-changer. Knowing the specific cause allows for targeted interventions.
- Identification of the Vector (Rat Flea – 1898): Paul-Louis Simond’s conclusive demonstration of the rat flea’s role in transmission from rodents to humans provided the crucial missing piece. This shifted public health strategies from vague “miasmas” to specific pest control.
These discoveries moved plague control from ineffective and often draconian measures to evidence-based interventions.
- Improved Public Health and Sanitation (Early 20th Century)
- Targeted Rat Control: Once the vector was understood, efforts focused on reducing rat populations in urban areas, especially in ports. This included better waste management, rat-proofing of buildings, and, later, the use of rodenticides.
- Flea Control: With the development of effective insecticides, particularly DDT in the mid-20th century, direct flea control became possible. Spraying efforts targeted areas where fleas were prevalent, further breaking the transmission cycle.
- Stricter Quarantine Measures: While quarantines existed before, advances in scientific understanding of plague enabled more effective, targeted protocols for ships and incoming goods, focusing on fumigation and rodent inspection.
- Improved Urban Infrastructure: Gradual improvements in urban sanitation, sewage systems, and housing quality in many cities (especially in Europe and North America) made environments less hospitable for rats and their fleas in close proximity to humans.
- Development and Widespread Use of Antibiotics (Mid-20th Century)
- Specific and Highly Effective Treatment: This was the single most impactful factor in reducing mortality. The discovery of streptomycin in the 1940s, followed by other antibiotics like tetracyclines and chloramphenicol, provided a highly effective cure for plague.
- Dramatic Drop in Case Fatality Rates: Before antibiotics, the mortality rate for bubonic plague was 50-90%. With early antibiotic treatment, this plummeted dramatically, often to less than 10-15%. For pneumonic plague, previously almost universally fatal, antibiotics also offered a chance of survival.
- Interrupting Transmission: By rapidly curing infected individuals, antibiotics also helped to stop human-to-human transmission (especially of pneumonic plague) and reduce the overall burden of infection in communities.
- Shift in Rat Species (Contributing Factor in Some Regions)
- In some parts of Europe, it’s hypothesized that the brown rat (Rattus norvegicus), which tends to live in sewers and basements rather than in close proximity to humans, largely replaced the black rat (Rattus rattus), which nested in human dwellings. This shift could have reduced the opportunities for human-flea-rat interactions. While debated as a sole cause, it may have contributed to the decline in some areas.
- Decline in Reintroduction Events (in Europe)
- While plague established new sylvatic (wild rodent) reservoirs in some areas (e.g., Western US and parts of Africa), repeated reintroductions of plague from Central Asian reservoirs into European ports likely diminished over time; this could be due to changes in trade routes or possibly ecological shifts in the source regions that reduced the “spillover” of plague into global trade.
By the mid-20th century, the combination of a scientific understanding of the disease, targeted public health interventions (especially rat and flea control), and the advent of highly effective antibiotic treatments effectively ended the Third Pandemic as a widespread human threat. While plague still exists in endemic foci globally, and sporadic human cases occur, it no longer causes the massive epidemics seen during the three great pandemics.
Bubonic Plague Modern Status
The modern status of the Bubonic Plague is vastly different from its historical devastation, thanks to scientific advancements in understanding and treating the disease.
Here’s a summary:
- Not Eradicated, but Rare and Highly Treatable: Bubonic plague still exists, but it is not a pandemic threat in the modern world. It’s considered rare in most countries, and critically, it is highly treatable with antibiotics if diagnosed early.
- Endemic Regions (Natural Foci): Yersinia pestis, the bacterium that causes plague, circulates naturally in wild rodent populations and their fleas in specific geographic areas around the globe. These are often referred to as “natural foci” or “enzootic cycles.” Key regions where human cases occur include:
- Africa: The Democratic Republic of Congo, Madagascar, and Peru account for a significant majority of reported human cases worldwide.
- Asia: Some countries in Asia continue to report sporadic cases.
- Americas: In the United States, plague is found in rural and semi-rural areas of the western states, including northern New Mexico, northern Arizona, southern Colorado, California, southern Oregon, and far western Nevada. Cases also occur in parts of South America.
- Global Case Numbers: Worldwide, approximately 1,000 to 2,000 human cases of plague are reported annually to the World Health Organization (WHO), though this figure can fluctuate.
- Cases in the United States: In the U.S., plague is very rare. An average of 7 human plague cases are reported each year (ranging from 0 to 17 in recent decades). Over 80% of U.S. cases are the bubonic form.
- Transmission to Humans: Humans typically acquire plague through:
- The bite of infected fleas that have fed on infected rodents.
- Direct contact with infected animal tissues or fluids (e.g., handling sick or dead animals like rodents, rabbits, or even domestic cats that have caught infected rodents).
- Less commonly, inhaling infectious droplets from a person or animal with pneumonic plague (the lung form).
- Treatment: Modern antibiotics are highly effective against Yersinia pestis. If treatment begins promptly, ideally within 24 hours of symptom onset, the chances of survival are very high (around 90% for all forms of plague). Common antibiotics used include streptomycin, gentamicin, doxycycline, and ciprofloxacin.
- Prevention and Control: Public health efforts focus on:
- Surveillance: Monitoring rodent and flea populations in endemic areas for signs of plague activity.
- Rapid Response: Prompt investigation and treatment of human cases to prevent further spread.
- Public Education: Advising people in endemic areas on how to prevent exposure (e.g., avoiding contact with wild rodents, using flea control on pets, reducing rodent habitats around homes).
- Vaccination: A plague vaccine exists but is generally not recommended for the general public due to the rarity of the disease. It’s primarily used for high-risk groups, such as laboratory personnel who work with the bacteria or military personnel deployed to active plague areas.
In summary, while the bubonic plague no longer poses the same widespread threat as it did during historical pandemics, it remains a zoonotic disease that continues to circulate naturally in certain ecosystems. Thanks to modern science and medicine, it is now a manageable and treatable illness when detected early.
Smallpox
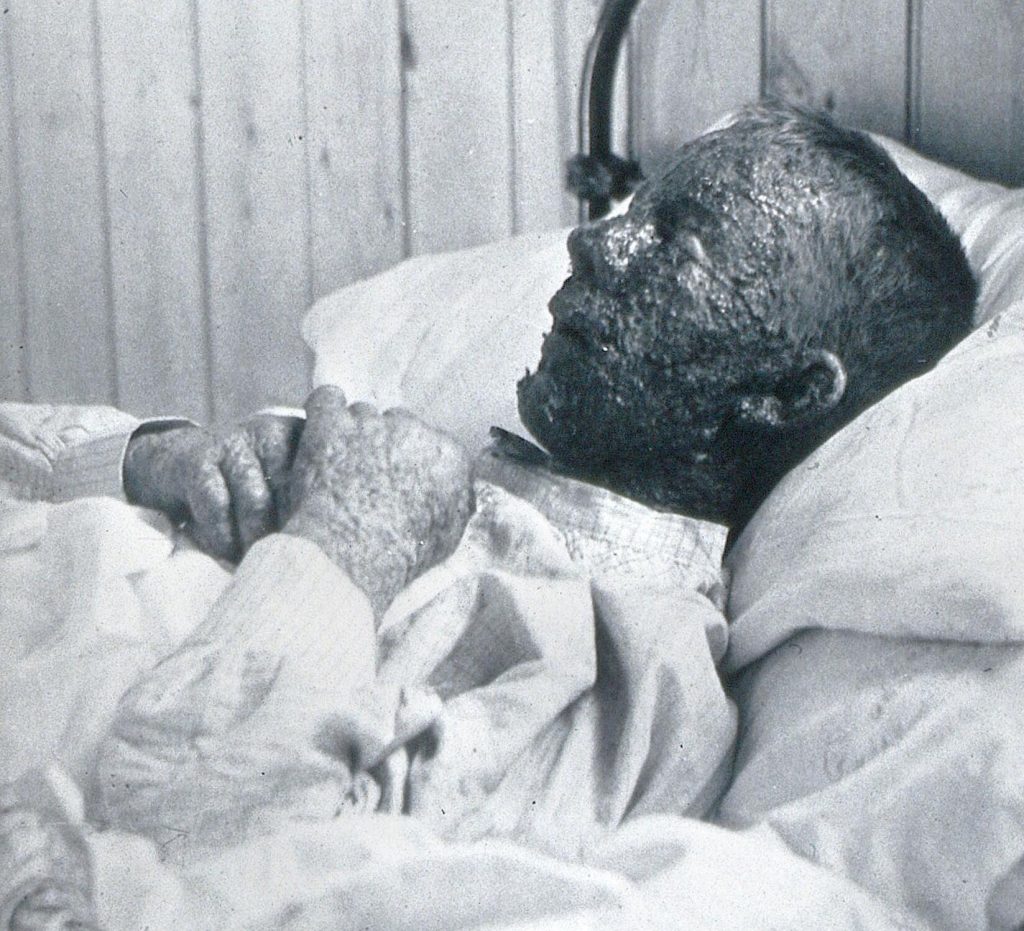
Malignant hemorrhagic smallpox in a baker during a 1896 epidemic in Gloucester, England. Died 8 days after admission.
(Wiki Image By H.C.F – https://wellcomecollection.org/works/y2xgarem, CC0, https://commons.wikimedia.org/w/index.php?curid=113649420)
Smallpox Quotes
Here are some powerful and historically significant quotes related to smallpox, highlighting its impact, the fear it inspired, and the triumph of its eradication:
On the Disease’s Impact and Fear:
- “Smallpox is the most terrible of all the ministers of death.” – Thomas Macaulay (19th-century British historian and politician), emphasizing the disease’s devastating power.
- “Among the destroyers of men, smallpox is the chief.” – Edward Jenner (18th-century English physician, pioneer of the smallpox vaccine), acknowledging the immense toll before his discovery.
- “The pestilence of smallpox, which had for so many centuries been filling the grave-yards of the world with countless millions of victims, and leaving the survivors disfigured and blinded, was now to disappear forever from the earth.” – Andrew Carnegie (19th-20th century American industrialist and philanthropist), looking back at the disease’s history after its eradication, capturing the sense of liberation.
- “Smallpox… has proved itself a more successful exterminator of aboriginal populations than gunpowder and firewater combined.” – Jared Diamond (Contemporary American geographer, historian, and author), from “Guns, Germs, and Steel,” highlighting its devastating impact on indigenous populations, especially in the Americas.
On Vaccination and Eradication:
- “Vaccination is the greatest discovery ever made.” – Edward Jenner, a testament to his own groundbreaking work and its potential.
- “The cow-pox is our security.” – Edward Jenner, referring to the origin of the vaccine from cowpox.
- “No country can claim freedom from smallpox until the whole world is free.” – Donald A. Henderson (20th-21st century American physician and epidemiologist, led the WHO’s smallpox eradication effort), emphasizing the global nature of the campaign and the interconnectedness of public health. This quote became a guiding principle for the eradication program.
- “The last person on earth to suffer from smallpox will have been born already.” – Donald A. Henderson, a prescient statement made during the final stages of the eradication campaign, signifying the approaching victory.
- “The eradication of smallpox remains the greatest triumph of medical science.” – World Health Organization (WHO), a widely recognized statement celebrating the achievement.
- “A magic bullet did not defeat smallpox, but by methodical, determined, global human will.” – Larry Brilliant (Contemporary American epidemiologist played a key role in the smallpox eradication program), emphasizing the human effort and collaboration involved.
These quotes collectively illustrate the journey from terror and despair to the immense pride and relief associated with the defeat of a historical Scourge.
Smallpox YouTube Video
-
- How we conquered the deadly smallpox virus – Simona Zompi | TED-Ed – 8,934,415 views
- History of vaccination: Smallpox vaccines | WHO European Region – 136,069 views
- Smallpox eradication : Science, solutions, solidarity | World Health Organization (WHO) – 19,340 views
- This is what Smallpox looks like! #meded #biology #anatomy | SciePro – 218,404 views
- 6 Vials of Small Pox Left Unguarded for Decades Found | ABC News – 33,222 views
Smallpox Books
Smallpox is one of the most historically significant diseases, being the only human disease ever to be successfully eradicated by a global campaign. The books on smallpox often fall into two categories: the harrowing history of its devastating impact, and the inspiring public health story of its final defeat.
Here is a selection of essential reading on the subject:
🔬 The Triumph of Eradication (Inside the WHO Campaign)
These books provide the definitive account of the World Health Organization (WHO) campaign that led to the eradication of smallpox.
-
Smallpox: The Death of a Disease: The Inside Story of Eradicating a Worldwide Killer by D. A. Henderson, M.D.
-
Significance: This is the foundational book on the eradication effort, written by the man who directed the global campaign from 1967 to 1977. Henderson provides a firsthand, behind-the-scenes account of the logistical, political, and cultural challenges faced by the WHO, including battling bureaucracy and the Cold War politics that influenced the project.
-
-
The Greatest Killer: Smallpox in History by Donald R. Hopkins
-
Significance: Hopkins, a key figure in the eradication campaign, provides a sweeping historical overview of the disease, tracing its origins in mummies to its final defeat. This book is often cited as the definitive resource on the cultural, medical, and social history of smallpox.
-
-
Smallpox and Its Eradication by Frank Fenner, D.A. Henderson, et al.
-
Significance: This monumental, technical work published by the WHO (1988) is the final, definitive historical and scientific record. Authored by the experts personally involved in the campaign, it meticulously recounts the history of the disease and the minute-by-minute account of the successful global eradication effort.
-
📜 Historical Impact and Narratives
These books focus on smallpox’s devastating impact on history, particularly in the Americas.
-
Pox Americana: The Great Smallpox Epidemic of 1775–82 by Elizabeth A. Fenn
-
Significance: This highly acclaimed book argues that the smallpox epidemic that raged during the American Revolutionary War was a crucial, often overlooked factor in the conflict’s outcome. It details the epidemic’s devastating impact on Indigenous populations and on the Continental Army.
-
-
The Speckled Monster: A Historical Tale of Battling Smallpox by Jennifer Lee Carrell
-
Significance: This book focuses on the early history of inoculation (before Jenner’s vaccine) and the dramatic role played by Lady Mary Wortley Montagu, who brought the practice from Turkey to England in the 18th century, despite intense public and medical opposition.
-
-
War Against Smallpox: Edward Jenner and the Global Spread of Vaccination by Michael Bennett
-
Significance: This book is an in-depth history of the early triumph of vaccination following Jenner’s discovery. It explores the global networks that spread the cowpox vaccine during the Napoleonic Wars and details the hopes, fears, and early vaccine hesitancy among populations worldwide.
-
Smallpox Science
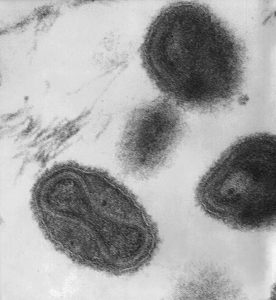
This transmission electron micrograph depicts several smallpox virions. The “dumbbell-shaped” structure inside the virion is the viral core, which contains the viral DNA; Mag. = ~370,000×
(Wiki Image By Content Providers(s): CDC/ Dr. Fred Murphy; Sylvia Whitfield – This media comes from the Centers for Disease Control and Prevention’s Public Health Image Library (PHIL), with identification number #1849.Note: Not all PHIL images are public domain; be sure to check copyright status and credit authors and content providers.العربية | Deutsch | English | македонски | slovenščina | +/−, Public Domain, https://commons.wikimedia.org/w/index.php?curid=1214308)
- Variola Virus:
- Smallpox Virus Structure:
- Smallpox Vaccine (Scientific):
Smallpox was one of the most devastating diseases in human history, causing millions of deaths and leaving survivors scarred or blinded. Its complete eradication in 1980 stands as one of humanity’s greatest scientific and public health triumphs. Here’s a look at the science behind smallpox:
- The Virus: Variola Virus
- Causative Agent: Smallpox is caused by the variola virus (VARV), a member of the Orthopoxvirus genus within the Poxviridae family.
- Structure: Variola virus is a large, brick-shaped virus with a complex structure. Unlike many other DNA viruses, it replicates in the host cell’s cytoplasm rather than its nucleus.
- Strains: There were two main clinical forms of smallpox:
- Variola major: The more common and severe form, with a mortality rate of up to 30% or higher.
- Variola minor: A milder form with a much lower mortality rate (less than 1%).
- Host Specificity: Crucially for its eradication, the variola virus infects only humans. There is no animal reservoir for the virus, meaning it couldn’t hide in animal populations and re-emerge. This characteristic was a key factor in the success of the eradication campaign.
- Transmission:
- Person-to-Person: Smallpox primarily spreads from person to person through direct and fairly prolonged face-to-face contact.
- Respiratory Droplets: The virus was transmitted through large respiratory droplets released when an infected person talked, coughed, or sneezed.
- Contaminated Objects: Less commonly, it could spread through direct contact with infected bodily fluids or contaminated objects like bedding or clothing (fomites).
- Airborne (Rarely): In rare instances, particularly in enclosed settings, smallpox could be transmitted by airborne particles over short distances.
- Contagious Period: An infected person was typically contagious from the onset of fever (prodrome phase), but became most contagious with the appearance of the rash. Contagion persisted until all the scabs had fallen off, which could take about 3 weeks.
- Pathogenesis and Symptoms:
- Incubation Period: After exposure, symptoms typically appeared after an incubation period of 10 to 14 days (ranging from 7 to 19 days), during which the person felt fine and was not contagious.
- Initial Symptoms (Prodrome): The illness began with flu-like symptoms, including:
- High fever (often sudden onset)
- Malaise (general feeling of unwellness)
- Severe headache
- Body aches (especially backache)
- Sometimes, vomiting or abdominal pain
- Rash Development:
- Early Rash: About 2-4 days after the initial symptoms, a characteristic rash emerged, first as small red spots on the tongue and in the mouth. These spots developed into sores that broke open, releasing large amounts of virus into the mouth and throat. This is when the person became most contagious.
- Skin Rash: Around the same time, a rash appeared on the skin, typically starting on the face and spreading to the arms, legs, and then to the hands and feet. The rash usually spreads to all parts of the body within 24 hours.
- Progression of Rash: The skin rash progressed through several stages over about 3-4 weeks:
- Macules: Flat, red spots.
- Papules: Raised bumps.
- Vesicles: Fluid-filled blisters.
- Pustules: Firm, pus-filled lesions.
- Scabs: Pustules crusted over, forming scabs.
- Scab Falling Off: The scabs would eventually fall off after about three weeks, often leaving pitted scars (pockmarks).
- Complications: Survivors often had permanent scarring, especially on the face. Smallpox could also lead to blindness (ocular complications) and, in severe cases, multi-organ failure.
- Diagnosis and Treatment:
- Diagnosis: Smallpox was typically diagnosed clinically based on its distinctive rash. Laboratory confirmation involved identifying the variola virus in samples such as blister fluid, skin scrapings, or blood.
- Treatment: There was no specific cure for smallpox. Treatment was primarily supportive, focusing on managing symptoms, providing intravenous fluids, controlling fever and pain, and treating any secondary bacterial infections.
- Antivirals (Post-Eradication): Following eradication, research has led to the development and FDA approval of antiviral drugs, such as tecovirimat (TPOXX) and brincidofovir (TEMBEXA), for potential use in smallpox emergencies. These drugs work by inhibiting viral replication, though they were not tested in people with active smallpox because smallpox has been eradicated.
- Prevention and Eradication: The Scientific Triumph
- Variolation: Before vaccination, an early method of prevention was “variolation,” which involved inoculating healthy individuals with material from smallpox pustules. While it provided some immunity, it carried risks, as it used the actual smallpox virus and could cause a severe case or spread the disease.
- Jenner’s Vaccine: The scientific breakthrough came in 1796 with Edward Jenner’s discovery of the smallpox vaccine. He observed that milkmaids who had contracted cowpox (a milder, related virus) were immune to smallpox. By inoculating people with cowpox material, he found he could protect them from smallpox. This was the first successful vaccine and the origin of the term “vaccination” (from vacca, Latin for cow).
- The Vaccine Mechanism: The smallpox vaccine used the vaccinia virus, another orthopoxvirus that is less harmful to humans than variola but elicits an immune response that cross-protects against smallpox.
- Global Eradication Campaign (WHO): The World Health Organization (WHO) launched an intensified global eradication program in 1967, led by figures like Donald A. Henderson. Key scientific and strategic elements of this campaign included:
- Freeze-dried Vaccine: Development of a heat-stable, freeze-dried vaccine that could be transported and stored in tropical climates.
- Bifurcated Needle: A simple, inexpensive, and efficient two-pronged needle that allowed for easy and standardized vaccination.
- Surveillance and Containment (Ring Vaccination): This was the cornerstone of the eradication strategy. Instead of mass vaccination of entire populations, public health workers focused on:
- Case Detection: Rapidly identifying new cases.
- Isolation: Separating infected individuals to prevent the spread of infection.
- Contact Tracing: Identifying all individuals who have come into contact with the infected person.
- Ring Vaccination: Vaccinating all contacts and their contacts (a “ring” of immunity) around each new case. This targeted, localized approach effectively broke transmission chains.
- Last Cases: The last naturally occurring case of variola major was in Bangladesh in 1975, and the last naturally occurring case of variola minor was in Somalia in 1977. Smallpox was officially declared eradicated by the WHO in 1980.
- Legacy: The eradication of smallpox is a monumental achievement, demonstrating the power of scientific research, vaccine development, and global public health cooperation to eliminate a deadly disease. It also provided a blueprint for future eradication efforts. Today, official stocks of variola virus are held in only two secure laboratories (one in the US and one in Russia) for research purposes related to antiviral development and vaccine improvement, primarily for biodefense preparedness.
Smallpox Epidemics. Table
Smallpox, caused by the variola virus, was one of the most historically significant diseases due to its widespread and devastating epidemics across millennia. While it’s now eradicated, its impact shaped human history, demography, and the course of medicine.
Here’s a table summarizing some of the most notable periods and impacts of smallpox epidemics:
Major Historical Smallpox Epidemics and Their Impact
| Time Period (Approximate) | Geographic Scope | Estimated Mortality / Impact | Key Characteristics & Significance |
| Ancient Origins (Pre-1st Millennium AD) | Northeastern Africa (e.g., Egypt), India, China | Evidence suggests significant mortality in early populations (e.g., Pharaoh Ramses V, 12th century BC). | Likely originated in Africa around 10,000 BC. Early descriptions in Chinese (4th century AD), Indian (7th century AD), and Mediterranean (7th century AD) texts. Periodically epidemic, causing high death tolls in unexposed populations. |
| Roman Empire & Europe (1st – 7th Centuries AD) | Roman Empire later spread throughout Europe | Antonine Plague (165-180 AD): Estimated 5-10 million deaths (25-33% of Roman population). While possibly smallpox or measles, it had a profound impact. <br> Spread intensified during major historical events. | Introduction to Europe likely via trade and military movements. The chronic, endemic presence, coupled with frequent devastating outbreaks, contributes to societal decline and depopulation. |
| Japanese Smallpox Epidemic (735-737 AD) | Japan | An estimated 1-2 million deaths (about 1/3 of the Japanese population). | A particularly severe outbreak had a significant impact on Japanese society, leading to political instability and contributing to changes in government policy and religious practices. |
| Age of Exploration & The Americas (16th-19th Centuries) | Americas, Pacific Islands (e.g., Hawaii, Australia) | Mexican Epidemic (1520): 5-8 million deaths (40% of the population). <br> Great Plains Epidemic (1837): Decimated Native American tribes. <br> Overall, an estimated 90% mortality in some indigenous populations lacking prior immunity. | European colonizers unintentionally introduced smallpox (and other diseases) to virgin soil populations in the Americas, leading to catastrophic depopulation and the collapse of advanced civilizations (Aztecs, Incas). Instrumental in European conquest. |
| 18th Century Europe | Widespread across Europe | An estimated 400,000 deaths occur annually in Europe. One-third of survivors went blind. | Smallpox became endemic in most European countries, affecting all social classes, including monarchs (e.g., King Louis XV of France). This fueled the search for a preventive measure, leading to variolation and, later, vaccination. |
| 19th – Early 20th Century Global Outbreaks | Worldwide (especially Asia, Africa, parts of Europe, and the Americas) | An estimated 300 million deaths globally in the 20th century alone, before eradication. | Despite Jenner’s vaccine (1796), widespread implementation took time. Outbreaks continued, particularly in less-vaccinated or more isolated communities. International efforts began to gain traction, leading to increased vaccination coverage. |
| The Global Eradication Campaign (1967-1980) | Primarily in South America, Africa, and Asia (where it was still endemic) | Significant reduction in cases, culminating in zero natural cases. | A testament to global scientific and public health collaboration. Characterized by aggressive surveillance, ring vaccination, and containment strategies. The last natural case was in Somalia in 1977. |
| Official Eradication | Worldwide | Zero cases | Declared eradicated by the World Health Organization on May 8, 1980. The only human infectious disease to be completely wiped out. |
Export to Sheets
This table highlights how smallpox transitioned from an ancient endemic disease to a global pandemic force, particularly with the expansion of European colonization, and was subsequently systematically targeted and eradicated through a monumental public health effort.
Smallpox History
Drawing accompanying text in Book XII of the 16th-century Florentine Codex (compiled 1555–1576), showing Nahuas of conquest-era central Mexico with smallpox
(Wiki Image By en:Bernardino de Sahagún (1499-1590), compiler. Original illustration by an unknown 16th-century artist; this version of the drawing is by an unknown 16th-century copyist. – Florentine Codex (1540-1585), Book XII folio 54 [detail].As reproduced in: Fields, Sherry (2008). Pestilence and Headcolds: Encountering Illness in Colonial Mexico, Gutenberg-e series, e-book edn. New York: Columbia University Press, ISBN 978-0-231-14240-3.Reproduction from the MS. held at Biblioteca Medicea Laurenziana, Florence, Public Domain, https://commons)
- Smallpox History:
- Smallpox Symptoms (Historical):
- Smallpox Vaccine (Historical):
Smallpox was one of the most devastating diseases in human history, responsible for countless deaths and disfigurements over thousands of years. It holds the unique distinction of being the first (and to date, only) human infectious disease to be globally eradicated.
Early Origins and Ancient History
- Ancient Evidence: The exact origin of the variola virus (the cause of smallpox) is debated, but genetic evidence suggests it emerged 3,000 to 4,000 years ago. The earliest physical evidence comes from Egyptian mummies, notably the preserved head of Pharaoh Ramses V (who died in 1156 BCE), which bears characteristic smallpox lesions.
- Early Written Descriptions: The earliest written descriptions of a disease resembling smallpox appeared in China in the 4th century CE. Descriptions also surfaced in India in the 7th century and in Asia Minor (modern-day Turkey) by the 10th century.
- Early Spread: Smallpox likely spread from its early endemic regions (possibly the Nile Valley or Indus Valley) along ancient trade routes and with the movements of people, becoming established in densely populated parts of Eurasia and North Africa by the end of the first millennium CE.
Global Dissemination and Devastation
- Medieval Europe: The Crusades (11th-13th centuries) significantly contributed to the dissemination of smallpox throughout Europe. By the 1500s, it was endemic in most European countries, causing widespread illness and death, affecting all levels of society, including monarchs like Queen Elizabeth I of England (who survived but was disfigured in 1562).
- The Americas (16th Century): The arrival of Europeans in the Americas in the 16th century brought catastrophic consequences for indigenous populations who had no natural immunity. Smallpox was unintentionally introduced by Spanish conquistadors around 1520 and played a major role in the demise of the Aztec and Inca empires, killing millions of native inhabitants. It also spread throughout Central and South America via the slave trade from West Africa.
- 18th Century Peak: By the mid-18th century, smallpox was a major global endemic disease. It’s estimated to have killed around 400,000 Europeans annually and was responsible for about one-third of all cases of blindness.
Early Control Efforts: Variolation
- Ancient Practice: Long before vaccination, a method called variolation was used to provide some protection. This involved exposing uninfected individuals to material from smallpox sores (pustules), typically by scratching it into the skin or inhaling it.
- Spread to Europe: Variolation was practiced in China (as early as the 10th century), India, and parts of Africa (e.g., the Ottoman Empire). It was introduced to Europe in the early 18th century by figures like Lady Mary Wortley Montagu, wife of the British ambassador to Turkey.
- Risks: While variolation was less deadly than natural infection (with a fatality rate of about 0.5-2% compared to 30%), it still carried risks. Individuals could become very ill, develop a full-blown case, or even transmit the disease to others, initiating new outbreaks.
The Dawn of Vaccination
- Edward Jenner (1796): The breakthrough came in 1796 with English physician Edward Jenner. He observed that milkmaids who had previously contracted cowpox (a milder, related disease) seemed immune to smallpox. Jenner famously inoculated James Phipps, an 8-year-old boy, with material from a cowpox lesion on a milkmaid’s hand. After later exposing Phipps to the variola virus, Phipps did not develop smallpox, confirming his immunity.
- “On the Origin of the Vaccine Inoculation” (1801): Jenner published his findings, calling his method “vaccination” (from vacca, Latin for cow). This safer and more effective technique gradually replaced variolation.
- 19th-Century Progress: Vaccination gained wider acceptance, and by the mid-19th century, some states began mandating vaccination for schoolchildren, resulting in a progressive decline in the disease’s incidence, although severe epidemics continued to occur.
The Eradication Campaign
- WHO Initiative (1959): The World Health Organization (WHO) formally began planning for smallpox eradication in 1959. Early efforts faced challenges due to a lack of resources and commitment.
- Intensified Eradication Programme (1967): Under the leadership of D.A. Henderson and with strong support from the US and USSR, WHO launched the Intensified Smallpox Eradication Programme in 1967. Key strategies included:
- Mass Vaccination: Initial efforts focused on vaccinating large populations, often using new tools like the ped-o-jet injector.
- Surveillance and Containment (Ring Vaccination): This became the cornerstone strategy. Public health workers actively searched for smallpox cases, isolated infected individuals, and then vaccinated everyone in a “ring” around the case and their contacts. This localized approach prioritized resources and broke chains of transmission.
- Bifurcated Needle: The introduction of the bifurcated needle (a simple, inexpensive tool that delivered a precise dose of vaccine with minimal training) greatly accelerated vaccination efforts, particularly in remote areas.
- Final Cases:
- The last naturally occurring case of Variola major (the more severe form) was diagnosed in Rahima Banu in Bangladesh in October 1975.
- The last naturally occurring case of Variola minor (a milder form) occurred in Ali Maow Maalin in Somalia in October 1977.
- The last fatal case globally was a laboratory-acquired infection in England in 1978.
- Official Eradication (1980): On May 8, 1980, the World Health Assembly officially declared smallpox globally eradicated, a monumental triumph of international public health collaboration.
Today, controlled stocks of the variola virus are kept in two high-security laboratories: one at the Centers for Disease Control and Prevention (CDC) in Atlanta, USA, and another at the State Research Center of Virology and Biotechnology (VECTOR) in Koltsovo, Russia. The smallpox vaccine is no longer routinely administered to the general public.
Smallpox Early Origins and Ancient History
Smallpox, a disease caused by the Variola virus, has a deep and devastating history, extending back millennia into ancient civilizations. Its early origins and spread laid the groundwork for its later catastrophic global impact.
Earliest Known Evidence (3,000 to 4,000 Years Ago):
- Archaeological Evidence: The earliest physical evidence of smallpox comes from ancient Egypt. Distinctive smallpox-like lesions have been identified on the mummy of Pharaoh Ramses V, who died around 1156 BCE. This finding suggests the disease was present in ancient Egypt at least 3,000 years ago. Other mummies from the 18th and 20th Egyptian Dynasties (1570–1085 BC) also show similar skin lesions. Genetic evidence from recent studies of ancient variola virus strains further supports an origin dating back over 3,800 years, consistent with historical hypotheses.
- Early Written Records: While physical evidence points to earlier existence, reliable written descriptions of a disease resembling smallpox appeared later:
- China: The earliest known written accounts describing a disease similar to smallpox date back to the 4th century CE.
- India: Descriptions can be found in ancient Sanskrit texts, with later, more reliable accounts from the 7th century CE.
- Asia Minor (present-day Turkey): Smallpox descriptions appear around the 10th century CE.
Ancient Spread and Epidemics:
- Northeast Africa and India: It is believed that smallpox originated in northeastern Africa and spread to India via ancient trade routes and merchant activity.
- Into Europe: Smallpox was introduced to Europe sometime between the 5th and 7th centuries CE. It became a frequent epidemic during the Middle Ages, significantly impacting the development of Western civilization.
- The Antonine Plague (165–180 CE), which devastated the Roman Empire and caused millions of deaths, is speculated by many historians to have been smallpox, contributing to the empire’s decline.
- Across Africa and Asia: By the 6th century CE, the highly contagious disease had spread extensively across Africa and Asia.
- Introduction to Japan: Increased trade with China and Korea introduced smallpox into Japan by the 6th century CE, leading to severe epidemics, such as the one in 735–737 CE, which killed a significant portion of the Japanese population.
- Further European Spread: The Crusades (11th-13th centuries) contributed significantly to the spread of smallpox within Europe, as Crusaders traveled between the Middle East and their homelands. By the 13th century, population expansion and increased travel had made smallpox endemic in Central and Northern Europe.
Throughout this ancient history, smallpox was a formidable opponent, often fatal, and left survivors with disfiguring scars. Knowledge of its contagious nature and the observation that survivors were immune led to early, crude attempts at prevention, such as variolation (deliberate exposure to mild forms of the disease), practiced in various forms in Africa, India, and China centuries before its introduction to Europe in the 18th century.
Smallpox Global Dissemination and Devastation: Medieval Europe
Smallpox, while originating much earlier, became a devastating force in Medieval Europe, contributing significantly to the era’s widespread disease burden and shaping its societal landscape. Its dissemination was closely tied to the movements of people, trade, and conflict during this period.
Arrival and Establishment in Europe: Smallpox is believed to have been introduced to Europe by the 5th or 6th century CE, possibly via trade routes from Africa or Asia. By the 7th century, it had become endemic throughout the continent, meaning it was consistently present within the population, causing regular outbreaks.
The Medieval Environment: A Perfect Breeding Ground: Medieval Europe’s conditions were ideal for the spread of highly contagious diseases like smallpox:
- Growing Urbanization: As towns and cities expanded, population density increased, bringing more people into close proximity and facilitating the transmission of airborne diseases.
- Poor Sanitation: Lack of adequate sanitation, waste disposal, and clean water sources in urban centers created environments where diseases could thrive.
- Frequent Travel and Trade: Although not as extensive as those of later periods, trade routes and pilgrimage paths facilitated the virus’s movement between communities.
- Warfare and Crusades: Large movements of armies and crusaders across continents facilitated the rapid dissemination of diseases. The Crusades (11th-13th centuries), in particular, are thought to have brought smallpox and other diseases back from the Middle East to Europe, further entrenching it.
Devastation in Medieval Europe: Smallpox was a constant threat, especially to children and those who had not yet contracted it.
- Endemic Childhood Disease: For centuries, it has been a common childhood disease. Those who survived often bore lifelong scars (“pockmarks”), and many were left blind. It was responsible for a significant portion of child mortality.
- High Mortality Rates: While specific mortality figures for medieval smallpox are hard to isolate from other diseases of the time, it was known to kill a substantial percentage of those infected, particularly affecting infants and young children. Death rates in epidemics could be as high as 20-60%.
- Impact on Royalty and Nobility: Smallpox spared no one, affecting even royal families and nobility. Its distinctive scarring made it easily identifiable, and its devastating impact on high-profile individuals underscored its reach.
- Concurrent with Other Plagues: Smallpox often raged alongside other infectious diseases. While the Black Death (mid-14th century), caused by Yersinia pestis, was the dominant pandemic of the era, smallpox continued its endemic toll, contributing to the overall high mortality and short life expectancy in medieval society. It weakened populations, potentially making them more susceptible to other illnesses.
By the end of the Medieval period and into the Renaissance, smallpox was a well-established and terrifying presence across Europe, laying the groundwork for its later catastrophic introduction to the Americas and its global devastation in subsequent centuries.
Smallpox Global Dissemination and Devastation: The Americas (16th Century)
The 16th century witnessed a catastrophic chapter in the history of smallpox, as the disease was introduced to the Americas by European explorers and colonizers, unleashing an unprecedented demographic disaster upon indigenous populations who had no prior immunity.
Introduction to the “Virgin Soil” Populations:
- Arrival in the Caribbean (Early 16th Century): Smallpox first arrived in the Americas with Spanish ships, primarily through the importation of enslaved Africans or infected Spanish sailors and colonists. The first documented smallpox epidemic in the Americas began on the island of Hispaniola in late 1518 (or 1507, according to some sources), rapidly spreading and wiping out entire tribes of the Taíno people, who had no natural resistance.
- Spread to the Mainland (1520s onwards): From the Caribbean islands, smallpox quickly jumped to the mainland. In 1520, an infected enslaved African or Spanish soldier, part of an expedition led by Pánfilo de Narváez (sent to apprehend Hernán Cortés), introduced the virus to what is now Mexico. This coincided directly with Cortés’s conquest of the Aztec Empire.
Unprecedented Devastation: The impact of smallpox on the indigenous populations of the Americas was catastrophic, leading to what epidemiologists refer to as a “virgin soil” epidemic. Unlike Europeans and Africans, who had centuries of exposure and some degree of inherited or acquired immunity, Native Americans had no prior exposure to the Variola virus and, thus, no immunity.
- Collapse of Empires:
- Aztec Empire: When smallpox reached the Aztec capital of Tenochtitlan in 1520, it decimated the population, including Emperor Cuitláhuac, who died from the disease. The epidemic severely weakened the Aztec resistance, killing millions, demoralizing the survivors, and destroying their social and military structures. When Cortés returned in 1521 to conquer the city, he found it ravaged by disease, allowing for a swift victory over a weakened and bewildered populace. Estimates suggest Mexico’s indigenous population fell from over 30 million before Cortés’s arrival to as few as 1.5-3 million by 1568, with smallpox being a primary driver.
- Inca Empire: Smallpox also spread rapidly through the highly organized Inca Empire in South America, even before Francisco Pizarro and the Spanish conquistadors fully arrived. It traveled along their efficient road system, killing Emperor Huayna Capac and his designated successor. This plunged the empire into a devastating civil war between two surviving brothers, significantly weakening it just as Pizarro launched his conquest in the 1530s. Smallpox alone is estimated to have claimed between 60% and 90% of the Inca population.
- Massive Mortality Rates: Across the Americas, mortality rates were staggeringly high, often reaching 50-90% or more in affected communities. Entire villages and tribes were wiped out, leading to widespread social collapse, famine (as there were too few healthy individuals to tend crops), and immense trauma.
- Facilitation of European Conquest: While European weaponry and tactics played a role, historians widely agree that diseases, especially smallpox, were the single most decisive factor in the rapid and devastating conquest of the Americas. The “invisible killer” cleared the way for European dominance, transforming the demographics and cultures of two continents forever.
The 16th-century smallpox epidemics in the Americas stand as a stark historical example of the devastating power of disease when introduced to immunologically naive populations.
Smallpox Global Dissemination and Devastation: 18th Century Peak
The 18th century marked a horrifying peak for smallpox’s global devastation, cementing its reputation as one of humanity’s most feared scourges before the advent of widespread vaccination. It was a period where the disease became a truly endemic and catastrophic presence across continents, particularly in Europe.
Smallpox’s Dominance in 18th-Century Europe:
- Massive Annual Death Tolls: In Europe alone, smallpox is estimated to have killed around 400,000 people annually throughout the 18th century. This made it arguably the single most lethal disease of the era in many regions, accounting for a significant percentage of all deaths (e.g., 6-10% of all burials in London).
- Childhood Scourge: By the 18th century, smallpox was firmly established as an endemic childhood disease in Europe. Most individuals contracted it during childhood, and while survivors gained lifelong immunity, the disease disproportionately affected the very young. In places like Russia, it was reported that every seventh child born died from smallpox.
- High Mortality and Morbidity: Of those infected, mortality rates ranged significantly but often hovered between 20% and 60%, with rates exceeding 80% in infected children. Survivors faced lifelong disfigurement from pockmarks, which was responsible for approximately one-third of all blindness in the era.
- Impact on Royalty: Smallpox did not discriminate by social class, claiming the lives of several reigning European monarchs in the 17th and 18th centuries, including Louis XV of France in 1774, Habsburg Emperor Joseph I, Queen Mary II of England, and Czar Peter II of Russia. These high-profile deaths highlighted the disease’s pervasive threat.
- Endemic Peaks: The disease was always present, but its incidence would peak every two to three years in urban centers like London, reflecting the constant cycle of new susceptible individuals (births) encountering the virus.
Global Spread Beyond Europe: The intense European maritime exploration, trade, and colonization efforts of the 18th century also ensured smallpox’s continued global reach and devastation:
- Continued Impact in the Americas: Although smallpox had decimated indigenous populations in the Americas in the 16th century, outbreaks continued throughout the 18th century among both Native Americans and colonial settlers, albeit with varying severity. For example, epidemics occurred during the American Revolutionary War (1775-1776), posing significant challenges for armies.
- Asia and Africa: Major smallpox outbreaks were recorded across Asia (e.g., in the Sultanates of Banjar and Tidore) and Africa (e.g., Cape Town in 1713, devastating Khoisan populations). These epidemics were often exacerbated by increasing contact with European colonists and traders, who unknowingly facilitated the virus’s spread.
- Arrival in Australia: Smallpox was introduced to Australia in the 18th century, with the first recorded outbreak in April 1789 (about 16 months after the First Fleet’s arrival), having a devastating impact on the Aboriginal population around Sydney Cove.
Despite the prevalence of smallpox during this period, the 18th century also saw the crucial development of variolation (a risky form of inoculation) becoming more widespread. This practice, originating in Asia and Africa, was adopted in Europe and provided some protection, though it remained hazardous. It ultimately set the stage for Edward Jenner’s groundbreaking development of the smallpox vaccine in 1796, which would eventually lead to the disease’s global eradication.
Smallpox Early Control Efforts: Variolation
Before the advent of modern vaccination, the earliest effective, albeit risky, method of controlling smallpox was a practice known as variolation. This technique, originating centuries ago, involved deliberately exposing an uninfected individual to material from a smallpox lesion to induce a milder, but still infectious, form of the disease.
Origins of Variolation: The exact origin of variolation is debated, but evidence suggests it emerged independently in various parts of the world, likely in China or India, possibly as early as the 10th century CE, and was also practiced in parts of Africa and the Middle East.
- China: In China, variolation was known as “sowing the pox” or “planting the smallpox.” Methods included grinding up smallpox scabs and blowing the powder into the nostrils or inserting cotton plugs soaked with variola matter into the nostrils.
- India: Practices in India involved scratching fluid from a smallpox pustule into the skin.
- Africa and the Middle East: Various forms of inoculation through skin scratches or inhalations were also recorded.
How Variolation Was Performed: The basic principle of variolation was to introduce a small amount of the Variola virus into a healthy person’s body, typically through a method less direct than natural infection, hoping to induce a milder case of the disease. Common methods included:
- Inoculation by Scratching: A small incision or scratch was made on the skin (often on the arm or leg), and pus or fluid from a fresh smallpox lesion (from a patient with a mild case) was rubbed into it.
- Nasal Inoculation (Insufflation): Dried and powdered smallpox scabs were blown into the nostrils.
Effectiveness and Risks: Variolation was a significant advancement over natural infection, but it was far from perfect and carried substantial risks:
- Effectiveness: Individuals who successfully underwent variolation developed immunity to smallpox. The mortality rate from variolation was significantly lower than that from natural smallpox infection. While natural infection might kill 20-60% of those infected, variolation generally results in death in about 1-2% of cases, though some estimates put it higher depending on the practitioner and method.
- Risks:
- Full-blown Smallpox: The primary risk was that the variolated individual could still develop a full, severe case of smallpox and die.
- Contagion: Variolated individuals became infectious and could spread the disease to others, potentially starting new epidemics. This was a major public health concern.
- Other Infections: The practice involved open wounds, carrying the risk of secondary bacterial infections.
Introduction to Europe (18th Century): Variolation was introduced to Western Europe in the early 18th century.
- Lady Mary Wortley Montagu: In 1717, Lady Mary Wortley Montagu, wife of the British ambassador to the Ottoman Empire, observed the practice in Constantinople. Having survived smallpox herself and seen its devastating effects, she became a passionate advocate for variolation upon her return to England, having her own children variolated.
- Cotton Mather and Zabdiel Boylston (America): In 1721, during a smallpox epidemic in Boston, Puritan minister Cotton Mather, having learned of variolation from his enslaved African man Onesimus, convinced physician Zabdiel Boylston to perform the procedure. Despite public opposition and controversy, Boylston’s efforts contributed to a reduction in mortality rates in Boston.
Despite its risks, variolation offered the first real hope against smallpox and saved countless lives over the centuries. It also demonstrated the principle that intentional exposure to a milder form of disease could confer immunity, laying the intellectual groundwork for Edward Jenner’s development of the much safer and more effective smallpox vaccine in 1796.
Smallpox The Dawn of Vaccination
The dawn of vaccination against smallpox represents one of the most pivotal moments in medical history, transitioning from the risky practice of variolation to a safe and universally effective method of disease prevention. This breakthrough is almost entirely attributed to the pioneering work of Edward Jenner in the late 18th century.
The Pre-Vaccination Landscape: Variolation’s Imperfections Before Jenner, variolation was the primary method of conferring smallpox immunity. While effective in reducing mortality compared to natural infection, it had significant drawbacks: it still caused a true (though often milder) smallpox infection, carried a risk of death (around 1-2%), and, crucially, variolated individuals were infectious. They could trigger new smallpox outbreaks in the community. The search for a safer, non-contagious method was paramount.
Jenner’s Insight: The Cowpox Connection Edward Jenner (1749–1823), an English country doctor in Berkeley, Gloucestershire, was keenly observant of local folklore. He noted the common belief among milkmaids that contracting cowpox—a relatively mild disease of cattle that could be transmitted to humans, causing pustules usually on the hands—somehow protected them from smallpox. This folk wisdom suggested a cross-immunity.
- The Key Experiment (May 14, 1796): Jenner decided to test this hypothesis. He took pus from a cowpox lesion on the hand of a milkmaid named Sarah Nelmes. He then inoculated James Phipps, an 8-year-old boy, and his gardener’s son by scratching the cowpox material into Phipps’s arm.
- Results of the Experiment: Young Phipps developed a mild fever, some discomfort, and a localized lesion where he was inoculated. After a few days, he recovered completely. Crucially, a few weeks later, Jenner deliberately inoculated Phipps with material from a human smallpox lesion. Phipps did not develop smallpox. He was immune.
The Birth of “Vaccination”: Jenner coined the term “vaccination” for his procedure, deriving it from the Latin word vacca, meaning “cow.” He published his findings in 1798 in his seminal work, An Inquiry into the Causes and Effects of the Variolae Vaccinae, a Disease Discovered in Some of the Western Counties of England….
Why Vaccination Was a Breakthrough:
- Safety: Unlike variolation, vaccination with cowpox material did not cause a dangerous smallpox infection. The vaccinia virus (the active agent in the cowpox material and the basis for the modern smallpox vaccine) was benign in humans.
- Non-contagious: The vaccinated individual was not contagious and could not spread smallpox to others, eliminating a major public health risk associated with variolation.
- Effective Immunity: Vaccination conferred robust, long-lasting immunity against smallpox.
Immediate Impact and Global Adoption: While initially met with skepticism and some opposition, the clear advantages of Jenner’s vaccination soon became apparent. By the early 19th century, vaccination spread rapidly across Europe and beyond. Napoleon ordered his troops to be vaccinated, and expeditions were sent to vaccinate populations in the Americas and Asia. National vaccination programs began to emerge.
Jenner’s discovery, born from careful observation and empirical testing, laid the foundation for the entire field of vaccinology. It provided humanity with the first truly effective weapon against a major infectious disease, setting the stage for the later global eradication of smallpox in the 20th century.
Smallpox The Eradication Campaign
The eradication of smallpox stands as one of humanity’s greatest public health triumphs, a testament to unprecedented global cooperation and scientific determination. This monumental achievement, led by the World Health Organization (WHO), resulted in the complete elimination of a disease that had plagued humankind for millennia.
Early Attempts and the WHO’s Initial Efforts: While smallpox vaccination had been available since Edward Jenner’s discovery in 1796, widespread, consistent global vaccination efforts were challenging. Sporadic campaigns had reduced incidence in some areas, but the disease continued to thrive in endemic pockets, particularly in developing nations.
In 1959, the World Health Organization (WHO) formally adopted a resolution to initiate a global smallpox eradication program. However, initial efforts were hampered by a lack of funding, insufficient commitment from member states, and inadequate supplies of effective vaccines. Progress was slow, and smallpox continued to cause millions of cases and hundreds of thousands of deaths annually.
The Intensified Eradication Program (IEP) – 1967: Recognizing the shortcomings, the WHO, under the leadership of American epidemiologist Donald A. Henderson, launched the Intensified Eradication Program (IEP) in 1967. Several crucial innovations and commitments characterized this renewed effort:
- Increased Funding and Political Commitment: The U.S. and other nations significantly increased their financial contributions and political backing.
- Heat-Stable Vaccine: A breakthrough came with the development of a heat-stable, freeze-dried vaccine. This was critical because previous vaccines required refrigeration, making them difficult to transport and store in remote, hot climates where smallpox was most prevalent.
- Bifurcated Needle: The invention of the bifurcated needle in 1965 by Dr. Benjamin Rubin revolutionized vaccine delivery. This simple, two-pronged needle required less vaccine per dose, was easy to use (even by non-medical personnel), and allowed for quicker, more efficient mass vaccination, leaving a distinctive scar as proof of vaccination.
- Surveillance-Containment Strategy: This was the cornerstone of the IEP. Instead of simply aiming for mass vaccination of entire populations (which was logistically impossible in many areas), the strategy focused on:
- Surveillance: Actively seeking out every case of smallpox. This involved a network of thousands of health workers tracing rumors of outbreaks.
- Containment: Once a case was found, a “ring vaccination” approach was implemented. This meant vaccinating everyone in the immediate vicinity of the infected person (family, friends, neighbors) and those they had recently been in contact with, effectively creating a “firewall” around the outbreak. Isolation of the infected individual was also crucial.
Challenges Faced: The campaign faced immense challenges:
- Logistics: Reaching remote villages, navigating difficult terrain, and transporting vaccines to millions of people.
- Superstition and Resistance: Overcoming local beliefs, distrust of outsiders, and cultural barriers.
- War and Political Instability: Campaigns often had to operate in regions plagued by conflict, such as the Horn of Africa.
- Identifying Cases: The constant search for new cases required meticulous investigation and often involved incentives for reporting.
Key Milestones and Final Success: The IEP systematically moved across continents, eliminating the disease region by region:
- South America: Smallpox was eliminated from South America in 1971.
- Asia: By 1975, smallpox had been largely contained to the Horn of Africa and South Asia. The last case in Asia was in Bangladesh in 1975.
- Africa: The final stronghold was in the Horn of Africa, particularly Somalia. The last naturally occurring case of Variola major (the more common and severe form) in the world was diagnosed in Ali Maow Maalin, a hospital cook in Merca, Somalia, on October 26, 1977. He survived.
- The last known case of the milder Variola minor occurred in October 1975 in Bangladesh.
On May 8, 1980, following a global commission’s review, the World Health Assembly officially declared smallpox eradicated, making it the first and only human infectious disease to be completely wiped out from the face of the Earth. The achievement required the dedicated efforts of hundreds of thousands of health workers, scientists, and communities worldwide. Remaining live virus samples are held under strict containment in two high-security laboratories: one at the Centers for Disease Control and Prevention (CDC) in Atlanta, USA, and another at the State Research Center of Virology and Biotechnology VECTOR in Koltsovo, Russia.
Tuberculosis (TB)
Chest X-ray of a person with advanced tuberculosis: Infection in both lungs is marked by white arrowheads, and black arrows mark the formation of a cavity.
(Wiki Image By Unknown author – This media comes from the Centers for Disease Control and Prevention’s Public Health Image Library (PHIL), with identification number #2543.Note: Not all PHIL images are public domain; be sure to check copyright status and credit authors and content providers.العربية | Deutsch | English | македонски | slovenščina | +/−, Public Domain, https://commons.wikimedia.org/w/index.php?curid=978580)
Tuberculosis Quotes
Here are some powerful quotes about tuberculosis, reflecting its historical impact, the suffering it caused, and the hope and challenges of its treatment:
On the Devastation and Suffering of TB:
- “Consumption [tuberculosis] is a disease peculiar to the human race, and it has caused more havoc and destruction than all the wars, plagues, and famines combined.” – Robert Koch (German physician and microbiologist, who identified Mycobacterium tuberculosis as the cause of TB), highlighting its immense historical toll.
- “This pale-faced scourge, that has wasted more lives than all the wars and epidemics put together.” – Anton Chekhov (Russian playwright and physician, who died of TB), conveying the relentless and pervasive nature of the disease.
- “The disease that takes the most beautiful, the most gifted, and the most sensitive.” – A common sentiment or observation during the Romantic era, reflecting the tragic loss of artists and intellectuals to TB, leading to the romanticized, yet grim, image of the “consumptive.”
- “The white plague.” – A common historical term for tuberculosis, referring to the paleness often associated with the disease and its widespread nature.
- “Consumption…was so often associated with youth and beauty, and often with genius. It was a disease that refined the patient, gave him a spiritual look, made him a fit subject for a poem or a novel.” – Susan Sontag (American writer and critic), from “Illness as Metaphor,” critiquing the romanticized view of TB and exposing its grim reality.
On the Hope, Treatment, and Ongoing Struggle:
- “The greatest challenge to health is the resurgence of old diseases, like TB, and the emergence of new ones, like AIDS.” – Nelson Mandela (Former President of South Africa, advocate for TB awareness), emphasizing the persistent threat of TB even in modern times.
- “TB is a disease of poverty. It breeds in crowded, poorly ventilated slums, and it spreads rapidly among people whose immune systems are weakened by malnutrition and other infections.” – A paraphrasing of a common public health perspective, highlighting the social determinants of TB.
- “TB is curable, but only if we reach everyone who needs treatment.” – A common rallying cry in global public health campaigns, underscoring the importance of diagnosis and adherence to treatment.
- “The fight against tuberculosis is not just a medical battle, but a social and economic one as well.” – A perspective often shared by public health experts, acknowledging the broader factors contributing to TB.
- “Until everyone with TB is diagnosed and treated, the battle is not won.” – A modern public health message emphasizing the need for comprehensive efforts.
From Literature and Personal Accounts:
- “All night long the coughs ring out, the horrible coughs… and now and then a sigh, or a groan, or a whisper, and then, silence.” – George Orwell (English novelist who suffered from TB), from “Down and Out in Paris and London,” a stark depiction of life in a TB ward.
- “When you have TB, you are not living a life. You are just waiting to die.” – A quote often attributed to a patient, conveying the profound despair and hopelessness that the disease can inflict without treatment.
These quotes collectively illustrate the historical terror of TB, its intimate connection with social conditions, and the ongoing global commitment to its elimination despite the many challenges.
Tuberculosis YouTube Video
-
- What makes tuberculosis (TB) the world’s most infectious killer? – Melvin Sanicas | TED-Ed – 2,644,181 views
- 5 Things to Know About TB | Centers for Disease Control and Prevention (CDC) – 967,798 views
- Tuberculosis | Ninja Nerd – 564,850 views
- How Did Tuberculosis Get So Bad? #science #scishow #tuberculosis #health | SciShow – 479,913 views
- What is tuberculosis? | Provincial Health Services Authority (PHSA) – 16,329 views
Tuberculosis Books
Tuberculosis (TB), historically known as “consumption” or the “White Plague,” has had a profound influence on art, literature, and medicine. Books about TB often explore this duality: its romanticized cultural history versus its devastating medical reality.
Here is a selection of essential reading on the history and modern crisis of Tuberculosis:
📜 Classic & Cultural History
These books explore how TB shaped society, culture, and medicine, focusing on the era before effective treatments were widely available.
-
The White Plague: Tuberculosis, Man and Society by René and Jean Dubos
-
Significance: This is considered the foundational classic on the cultural history of TB, first published in 1952. The Dubos brothers masterfully trace the disease from its suspected ancient origins through the 19th-century Romantic period (when consumption was often falsely linked to artistic genius and delicacy) to the age of bacteriology.
-
-
Spitting Blood: The History of Tuberculosis by Helen Bynum
-
Significance: A comprehensive, modern social history that covers the entire span of the disease. Bynum explores the science and the public health response, detailing the rise of the sanatorium movement and the eventual triumph (and later complacency) of antibiotics.
-
-
Tuberculosis: The Great White Plague by Thomas Dormandy
-
Significance: This is an exhaustive, highly readable history that focuses on the medical, social, and political responses to the disease. Dormandy traces the narrative from Hippocrates’ early clinical descriptions to the eventual development of the BCG vaccine.
-
🎨 TB in Literature and Art
TB was a central theme in many famous novels and plays, often symbolizing doomed love, moral decline, or artistic sensitivity.
-
La Dame aux Camélias by Alexandre Dumas fils
-
This 1848 novel (which inspired Verdi’s opera La Traviata) is the definitive work of Romantic literature featuring consumption. The tragic heroine, Marguerite, is a beautiful courtesan who slowly succumbs to the disease, emphasizing the theme of life cut short by cruel fate.
-
-
The Magic Mountain by Thomas Mann
-
This towering 1924 modernist novel is set almost entirely in a Swiss sanatorium during the years leading up to World War I. While its protagonist is initially a visitor, the work uses the closed, sickly society of the sanatorium to explore the ideas, politics, and intellectual fervor of pre-war Europe.
-
💊 The Modern Crisis
This book addresses the alarming resurgence of TB and the challenges facing global public health today.
-
Catching Breath: The Making and Unmaking of Tuberculosis by Kathryn H. Mason
-
Significance: This book brings the story of TB into the 21st century. Mason provides a deep, sociological look at the modern crisis, focusing on the emergence of Multi-Drug Resistant Tuberculosis (MDR-TB), the failures of global health initiatives, and the ongoing human cost of the disease, particularly in developing nations.
-
Tuberculosis Science

Robert Koch discovered the tuberculosis bacillus.
(Wiki Image By Unknown author – https://ihm.nlm.nih.gov/images/B16691, Public Domain, https://commons.wikimedia.org/w/index.php?curid=6382628)
- Mycobacterium tuberculosis bacteria:
- Tuberculosis Diagnostic Methods:
- Tuberculosis Microscopic Images:
Tuberculosis (TB) is a serious infectious disease primarily caused by the bacterium Mycobacterium tuberculosis (M. tuberculosis). Despite being preventable and curable, it remains one of the top infectious killers globally. Understanding the science of TB is crucial for its control and eventual elimination.
- The Causative Agent: Mycobacterium tuberculosis
- Bacillus: M. tuberculosis is a rod-shaped bacterium (bacillus).
- Aerobic: It requires oxygen to grow.
- Slow-Growing: Compared to many other bacteria, M. tuberculosis is a slow-growing bacterium with a generation time of approximately 20 hours. This slow growth contributes to the long duration of TB treatment.
- Non-Motile and Non-Spore-Forming: It does not move on its own and does not form spores, which are dormant, highly resistant structures.
- Unique Cell Wall: A key characteristic is its complex, lipid-rich cell wall, which contains mycolic acids. This unique composition makes the bacterium:
- Acid-Fast: It resists decolorization by acid-alcohol after staining, a property used in rapid diagnostic tests (acid-fast bacilli or AFB smears).
- Impermeable: It makes the bacterium resistant to many common antibiotics and disinfectants and contributes to its survival within host cells.
- Virulence Factor: The cell wall components also play a role in modulating the host immune response and promoting the bacterium’s survival.
- Intracellular Pathogen: M. tuberculosis is a facultative intracellular pathogen, meaning it can survive and replicate inside host cells, particularly macrophages. This ability helps it evade the immune system.
- Transmission:
- Airborne Spread: TB is primarily spread through the air when a person with active pulmonary (lung) or laryngeal (throat) TB coughs, sneezes, speaks, or sings. These actions release tiny airborne particles called “droplet nuclei” containing M. tuberculosis bacteria.
- Inhalation: People nearby can breathe in these droplet nuclei and become infected.
- Not Spread by Fomites: TB is generally not spread by shaking hands, sharing food or drink, or touching contaminated surfaces.
- Pathogenesis (How the Disease Develops):
- Inhalation and Lung Infection: Once inhaled, the bacteria typically reach the alveoli (small air sacs) in the lungs.
- Macrophage Uptake: Macrophages, a type of immune cell, engulf the bacteria. However, M. tuberculosis has mechanisms to survive and multiply within these macrophages.
- Immune Response and Granuloma Formation: The immune system mounts a response, forming a specialized structure called a granuloma (also known as a tubercle) to wall off the bacteria. This granuloma is composed of infected macrophages, T cells, and other immune cells.
- Latent TB Infection (LTBI): In most healthy individuals (about 90-95%), the immune system successfully contains the bacteria within these granulomas. The person does not develop active TB disease, has no symptoms, and cannot spread the bacteria. This is known as latent TB infection. Individuals with LTBI have a positive TB test (skin or blood test) but a normal chest X-ray and no symptoms.
- Active TB Disease: In some individuals (about 5-10% of those with LTBI), especially those with weakened immune systems (e.g., HIV infection, malnutrition, diabetes, old age, certain medications), the contained bacteria can reactivate and multiply. This leads to the development of active TB disease, where the person becomes sick and can transmit the bacteria.
- Types of Active TB:
- Pulmonary TB: Most common form, affecting the lungs. Symptoms include persistent cough (often with sputum or blood), chest pain, fever, night sweats, weight loss, and fatigue.
- Extrapulmonary TB: Occurs when the bacteria spread from the lungs to other parts of the body, such as lymph nodes (scrofula), bones and joints (Pott’s disease of the spine), kidneys, brain (tuberculous meningitis), or other organs. Symptoms vary depending on the affected site.
- Miliary TB: A severe, widespread form where bacteria spread throughout the body via the bloodstream, leading to tiny lesions resembling millet seeds in multiple organs. This is more common in immunocompromised individuals.
- Diagnosis:
- TB Infection Tests:
- Tuberculin Skin Test (TST) / Mantoux Test: A small amount of tuberculin purified protein derivative (PPD) is injected into the skin. A positive reaction (induration) indicates exposure to TB bacteria.
- TB Blood Tests (Interferon-Gamma Release Assays or IGRAs): These tests (e.g., QuantiFERON-TB Gold Plus, T-SPOT.TB) measure the immune response to specific TB antigens in a blood sample. IGRAs are often preferred for people who have received the BCG vaccine, as BCG can cause a false positive TST.
- Diagnosis of Active TB Disease:
- Medical History and Physical Exam: Assessment of symptoms and risk factors.
- Chest X-ray (CXR): Helps identify lung abnormalities suggestive of TB (e.g., infiltrates, cavities, lymphadenopathy).
- Bacteriological Examination: This is crucial for definitive diagnosis and drug susceptibility testing:
- Sputum Smear Microscopy (AFB Smear): Rapidly identifies acid-fast bacilli in sputum samples. While quick, its sensitivity is limited.
- Nucleic Acid Amplification Tests (NAATs): Rapid molecular tests (e.g., GeneXpert MTB/RIF) that detect M. tuberculosis DNA and can simultaneously identify resistance to rifampicin (a key TB drug) within hours. This has revolutionized diagnosis, especially for drug-resistant TB.
- Culture: The “gold standard” for confirming TB diagnosis. Growing the bacteria from a sample (sputum, tissue, etc.) can take several weeks due to its slow growth, but it allows for definitive identification and comprehensive drug susceptibility testing.
- Drug Susceptibility Testing (DST): Performed on positive cultures to determine which anti-TB drugs the specific strain is susceptible or resistant to. Molecular DST can also be done rapidly.
- Treatment:
- Drug-Susceptible TB: Treatment for active TB involves a multi-drug regimen to prevent drug resistance and ensure cure. The standard regimen typically includes:
- Intensive Phase (2 months): Isoniazid (INH), Rifampicin (RIF), Pyrazinamide (PZA), and Ethambutol (EMB).
- Continuation Phase (4-7 months): Isoniazid and Rifampicin.
- Total Duration: 6 to 9 months, depending on the regimen and specific case.
- Adherence is Key: It is vital for patients to complete the full course of treatment, even if they feel better, to prevent the development of drug resistance and relapse.
- Latent TB Infection (LTBI) Treatment: Shorter regimens (e.g., 3 months of INH and rifapentine weekly or 4 months of daily rifampicin) are used to prevent LTBI from progressing to active TB disease.
- Drug-Resistant TB:
- Mechanism of Resistance: Drug resistance in M. tuberculosis arises primarily from genetic mutations in the bacterial DNA that alter the drug’s target or its ability to reach the target. This typically occurs when patients do not complete their full course of treatment or when inadequate drug regimens are prescribed.
- Multidrug-Resistant TB (MDR-TB): Defined as TB caused by strains resistant to at least the two most powerful first-line drugs: isoniazid and rifampicin. MDR-TB is much harder and more expensive to treat, requiring longer regimens (9-24 months) with more toxic second-line drugs.
- Pre-Extensively Drug-Resistant TB (Pre-XDR-TB): MDR-TB that is also resistant to any fluoroquinolone (a class of second-line drugs).
- Extensively Drug-Resistant TB (XDR-TB): Defined as MDR-TB that is resistant to a fluoroquinolone and at least one of bedaquiline or linezolid (two of the most effective newer drugs). XDR-TB is the most difficult form to treat, with very poor outcomes if not managed effectively.
- Research and Future Directions:
- New Diagnostics: Research is ongoing to develop faster, more accurate, and point-of-care diagnostic tests for both TB infection and drug resistance, particularly for use in resource-limited settings.
- New Drugs: The pipeline for new anti-TB drugs is improving, with new chemical entities and repurposed drugs in various stages of clinical trials. The goal is to develop shorter, safer, and more effective regimens, especially for drug-resistant TB.
- Vaccines: The only widely used TB vaccine, BCG (Bacille Calmette-Guérin), is effective in preventing severe forms of TB in children but offers limited protection against pulmonary TB in adults. Significant research is focused on developing new and more effective TB vaccines that can prevent infection or disease in adolescents and adults. Several vaccine candidates are in various phases of clinical trials.
- Host-Directed Therapies: Exploring therapies that target the host immune response to improve the body’s ability to clear the infection or reduce inflammation.
- Understanding Latency: Further research into the mechanisms by which M. tuberculosis can persist in a latent state is crucial for developing better preventive strategies.
Tuberculosis remains a complex global health challenge, driven by factors like poverty, HIV co-infection, and the rise of drug resistance. Continued scientific research and concerted public health efforts are crucial to achieving the goal of eradicating the TB epidemic.
Tuberculosis Epidemics. Table
Tuberculosis (TB) has been a constant companion to humanity for millennia, unlike acute epidemic diseases like plague or smallpox that caused sudden, sharp demographic collapses. Instead, TB has been a chronic, pervasive “silent killer,” particularly devastating during periods of social upheaval, urbanization, and poverty.
Here’s a table illustrating the major phases and characteristics of TB’s impact as an epidemic disease:
Historical Trends of Tuberculosis Epidemics
| Time Period (Approximate) | Geographic Scope | Impact & Characteristics | Key Contributing Factors | Medical / Societal Response |
| Ancient & Pre-Industrial Era (Neolithic to 17th Century) | Global, particularly in areas with dense human settlements. | Endemic presence. Paleopathological evidence in ancient remains (e.g., Egyptian mummies). A consistent cause of chronic illness and death. | Early human migration and close living conditions. Likely co-evolved with human populations. | Limited understanding of cause or transmission. Beliefs are often tied to divine punishment or inherited “consumption.” Folk remedies. |
| The “White Plague” Era (17th – 19th Centuries) | Europe and North America (especially urban centers) later spread globally with colonization. | Peak incidence and mortality: TB became a major epidemic, particularly in the 18th and 19th centuries. Accounted for 1 in 4 deaths in many European cities. Young adults were heavily affected, earning it names like “The White Plague” or “Captain of All These Men of Death.” | Industrial Revolution: Rapid urbanization, overcrowding, poor sanitation, malnutrition, and grueling working conditions created ideal breeding grounds for M. tuberculosis. Colonization: Spread to indigenous populations in the Americas, Africa, and Asia, who often had no prior immunity. | Development of sanatoriums (fresh air, rest, nutrition) as a form of “treatment.” Increased public awareness. Robert Koch identified M. tuberculosis in 1882. |
| Early 20th Century: Decline & Continued Burden | Western Europe and North America saw declines; the high burden continued in many other parts of the world. | Mortality rates began to fall in wealthier nations even before effective drugs due to improved living standards. Still a leading cause of death globally. | Improved Public Health: Better housing, nutrition, sanitation, and less crowded conditions in some regions. Public Health Campaigns: Early efforts at mass screenings (e.g., X-rays) and case finding. | Development of the BCG vaccine (limited effectiveness). The introduction of Streptomycin (1940s) and other antibiotics (1950s) revolutionized treatment, leading to a dramatic drop in cases and deaths in countries with access. |
| Late 20th Century: Resurgence & Drug Resistance (1980s-Present) | Global, but particularly acute in Sub-Saharan Africa, Southeast Asia, and Eastern Europe. | Resurgence: A “comeback” of TB in the 1980s, especially linked to the HIV/AIDS epidemic (TB is the leading cause of death for people with HIV). Drug-Resistant TB: Emergence and spread of multidrug-resistant TB (MDR-TB) and extensively drug-resistant TB (XDR-TB) due to inconsistent treatment, poor adherence, and inadequate drug regimens. Still a top infectious killer. | HIV/AIDS Pandemic: Weakened immune systems make individuals highly susceptible to TB and rapid progression to active disease. Inadequate TB Control Programs: Gaps in diagnosis, treatment adherence, and drug supply. Poverty & Inequality: Continued drivers of TB burden in low- and middle-income countries. | Development of new diagnostics (e.g., GeneXpert), new drugs (bedaquiline, delamanid), and shorter regimens for drug-resistant TB. Renewed global political commitment. |
| Current Situation (21st Century) | Global, with the highest burden in South-East Asia and Africa. | Ongoing Epidemic: Around 10 million people fall ill with TB annually, and 1.5 million die. A significant proportion of cases are drug-resistant. The COVID-19 pandemic disrupted TB services, leading to a temporary increase in cases and deaths. | Persistent social determinants (poverty, malnutrition, poor housing), co-epidemics (HIV, diabetes), limited access to rapid diagnostics and effective treatment, and the threat of antimicrobial resistance. | Global targets for TB elimination by 2030 (WHO End TB Strategy). Continued research into new vaccines, drugs, and diagnostics. Strengthening of national TB programs. |
Export to Sheets
Key Takeaways for TB Epidemics:
- Chronic vs. Acute: Unlike diseases that appear suddenly and then recede, TB has been a persistent, endemic presence, with its “epidemic” phases marked by increases in prevalence and mortality linked to societal conditions.
- Social Disease: TB is profoundly influenced by social and economic factors. Poverty, overcrowding, malnutrition, and poor healthcare access are major drivers of its spread and severity.
- Antibiotic Impact: The advent of antibiotics in the mid-20th century transformed TB from a death sentence to a curable disease in many parts of the world.
- Drug Resistance: The emergence of drug-resistant strains is a significant modern challenge, threatening to undo progress and making treatment much more complex and expensive.
- Global Burden: Despite significant declines in wealthier nations, TB remains a major global public health crisis, particularly in low- and middle-income countries.
Tuberculosis History
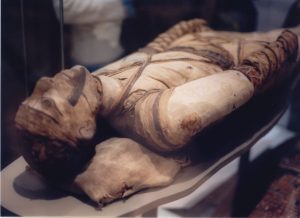
An Egyptian mummy in the British Museum has been found to have tubercular decay in the spine.
(Wiki Image CC BY-SA 3.0, https://commons.wikimedia.org/w/index.php?curid=15047)
- Tuberculosis History:
- Tuberculosis Sanatoriums (Historical):
- Tuberculosis Treatment (Historical):
Tuberculosis (TB), caused by the bacterium Mycobacterium tuberculosis, is one of the oldest and most persistent infectious diseases known to humanity. It has co-existed with humans for millennia, continuously adapting and profoundly shaping societies across different historical periods.
Here’s a chronological overview of its history:
Ancient Origins (Prehistory – 500 BCE)
- Deep Roots: Genetic evidence suggests that Mycobacterium tuberculosis evolved from an ancestral mycobacterium in East Africa millions of years ago, with the modern strains emerging around 70,000 years ago, co-evolving with early humans as they migrated out of Africa.
- Neolithic Era (c. 10,000 – 4,500 BCE): The earliest confirmed paleopathological evidence of human TB (skeletal lesions) dates back 9,000 to 11,000 years, found in Neolithic remains in the Near East. The transition from nomadic hunter-gatherer lifestyles to settled agricultural communities with increased population density and domesticated animals likely provided more favorable conditions for the bacterium’s spread.
- Ancient Civilizations: Evidence of TB (specifically spinal deformities known as Pott’s disease) has been found in Egyptian mummies dating back to 2400-3400 BCE. Ancient texts from India (c. 3300 years ago) and China (c. 2300 years ago) describe a wasting disease consistent with TB. The ancient Greeks referred to it as “phthisis” (meaning “to waste away”), and Hippocrates (c. 460 BCE) noted its widespread prevalence and often fatal outcome.
Medieval and Early Modern Eras (500 CE – 1800 CE)
- Middle Ages: TB remained widespread in Europe, often exacerbated by poor sanitation and crowded living conditions. A form of extrapulmonary TB affecting lymph nodes in the neck, known as “scrofula” or “king’s evil,” was common. There was a popular, superstitious belief that this could be cured by the “royal touch” of a monarch.
- Growing Understanding of Contagion: While its contagious nature was suspected for centuries (e.g., by the Arabian physician Avicenna in the 11th century and Girolamo Fracastoro in the 16th century), it wasn’t widely accepted. In the 17th century, physicians like Franciscus Sylvius began to provide more accurate pathological descriptions, identifying “tubercles” as the characteristic lesions.
- “The White Plague” / Consumption (17th – 18th Centuries): TB became known as “consumption” due to the dramatic weight loss of sufferers and “the white plague” for the pale complexion it often induced. It reached epidemic proportions, especially in crowded European cities during the early stages of the Industrial Revolution, becoming a leading cause of death. By the 18th century, it was responsible for approximately 25% of all deaths in Europe.
The Industrial Age and Scientific Breakthroughs (19th Century)
- Industrial Revolution Peak: The overcrowded, unsanitary conditions, poor nutrition, and long working hours of the Industrial Revolution provided a perfect breeding ground for TB, leading to its highest incidence rates. It devastated families and became a symbol of urban poverty.
- Naming the Disease: In 1839, Johann Lukas Schönlein officially coined the term “tuberculosis.”
- Proof of Contagion: In 1865, the French military doctor Jean-Antoine Villemin definitively demonstrated the infectious nature of TB by successfully transmitting it from humans to rabbits.
- Robert Koch’s Discovery (1882): The most significant breakthrough occurred on March 24, 1882, when German physician and microbiologist Robert Koch announced his discovery of Mycobacterium tuberculosis, the specific bacterium that causes the disease. This monumental discovery, for which he later won the Nobel Prize, provided a clear target for combating the disease and is commemorated annually on World TB Day.
- Sanatoria Movement: With the understanding of its contagious nature, the early 20th century saw the rise of sanatoria – specialized institutions for TB patients. Treatment focused on rest, fresh air, good nutrition, and gradually increased activity, as there were no effective drug cures yet. These institutions also served to isolate infected individuals to reduce spread.
The Modern Era: Treatment, Decline, and Resurgence (20th Century – Present)
- BCG Vaccine (1921): The Bacille Calmette-Guérin (BCG) vaccine, an attenuated form of Mycobacterium bovis, was developed by Albert Calmette and Camille Guérin in France. It was introduced in 1921 as a preventive inoculation, primarily for children, and has been widely used globally, though its effectiveness varies.
- Antibiotic Era (Mid-20th Century): The real turning point came with the discovery of effective antibiotics:
- Streptomycin (1944): Discovered by Selman A. Waksman and his team, this was the first effective antibiotic against M. tuberculosis.
- Isoniazid (1952): This drug proved to be even more effective and is still the cornerstone of TB treatment today.
- Other Drugs: Pyrazinamide (1954), ethambutol (1962), and rifampicin (1963) followed, enabling combination therapy that dramatically shortened treatment times and increased cure rates.
- Decline in Developed Countries: The widespread adoption of antibiotics, coupled with improved living standards, nutrition, and public health measures (like slum clearance and pasteurization of milk to prevent bovine TB), led to a steep decline in TB incidence and mortality in industrialized countries from the mid-20th century onwards.
- Resurgence and Global Emergency (1980s – Present): In the mid-1980s, TB rates began to rise again in developed countries due to several factors:
- Complacency: Reduced public health funding and surveillance.
- HIV/AIDS Epidemic: HIV severely weakens the immune system, making co-infected individuals highly susceptible to developing active TB. This fueled a massive global resurgence.
- Drug Resistance: The improper use of antibiotics (e.g., incomplete treatment courses) led to the evolution of drug-resistant strains, including Multi-Drug Resistant TB (MDR-TB) and Extensively Drug-Resistant TB (XDR-TB), which are much harder and more expensive to treat.
- Globalization and Migration: Increased travel and migration from high-burden countries also contributed to the spread.
- WHO Declaration (1993): In response to the growing crisis, the World Health Organization (WHO) declared TB a global emergency in 1993.
- Current Status: Today, TB remains a major global health challenge, particularly in low- and middle-income countries. It is one of the top infectious killers worldwide, responsible for approximately 1.5 million deaths annually. Efforts continue to improve diagnosis, develop new drugs and vaccines, and strengthen global control programs to ultimately eradicate the disease.
TB Ancient Origins (Prehistory – 500 BCE)
Tuberculosis (TB), caused by the bacterium Mycobacterium tuberculosis, is one of the oldest known human diseases, with its origins tracing back to prehistoric times and co-evolving with early human populations long before recorded history.
Deep Roots in Prehistory (70,000 – 10,000 Years Ago): Recent biomolecular and genetic studies suggest that human TB strains originated in early human populations in Africa at least 70,000 years ago. This predates the traditional theory that TB jumped from domesticated animals to humans during the Neolithic Revolution. Instead, it appears TB developed the ability to become dormant in its human hosts around 20,000 to 30,000 years ago, a crucial adaptation that allowed it to survive in small, dispersed hunter-gatherer populations. As Homo sapiens migrated out of Africa, TB migrated with them, adapting to various human groups across the globe.
Neolithic Period and the Rise of Agriculture (c. 10,000 – 4,000 BCE): The earliest confirmed paleopathological (study of ancient diseases) evidence of human TB in skeletal remains dates to the Pre-Pottery Neolithic (around 8,000 to 10,000 years ago), coinciding with the advent of agriculture and permanent settlements in the Near East. This “Neolithic demographic transition,” with its increased population density, sedentism, and closer proximity to livestock (even if human TB originated in humans), provided more favorable conditions for the transmission and maintenance of the disease.
Key archaeological sites with evidence of TB from this period include:
- Dja’de el Mughara and Tell Aswad (Syria): Remains from these sites, dating to 8800-7600 BCE, show lesions consistent with TB. Molecular analyses have confirmed the presence of M. tuberculosis DNA.
- Ain Ghazal (Jordan): Skeletons from this Neolithic village, dated to 7250 BCE, also suggest the presence of human TB.
- Atlit-Yam (Israel): Submerged Neolithic village dating from 6200-5500 BCE, where molecular analyses confirmed TB in the remains of at least two individuals (a presumed mother and son).
Ancient Civilizations (c. 4000 BCE – 500 BCE): As complex civilizations emerged, TB continued to be a significant health burden, leaving its mark in both skeletal remains and ancient texts.
- Ancient Egypt (from ~3400 BCE):
- Skeletal remains of Egyptian mummies, some dating back to as early as 3400 BCE, exhibit characteristic tubercular lesions, particularly in the spine (known as Pott’s disease).
- Many scholars also interpret tomb drawings and sculptures of hunchbacks as depictions of individuals suffering from spinal TB.
- Medical papyri, such as the Ebers Papyrus (c. 1550 BCE), describe lung ailments with symptoms similar to pulmonary TB and suggest treatments.
- Ancient India (from ~1500 BCE):
- The earliest references to TB in non-European civilizations are found in the Vedas. The oldest, the Rigveda (c. 1500 BCE), refers to the disease as “yaksma” (wasting disease).
- The Atharvaveda (c. 1500 BCE) names it “Balasa” and provides the first description of scrofula (TB of the lymph nodes). Ancient Ayurvedic texts also discuss its symptoms and treatments.
- Ancient China (before 500 BCE):
- The ancient Chinese medical work, the Huang Ti Nei-Ching (believed to originate from the 3rd millennium BCE), describes tuberculosis as a “wasting disease.”
- Ancient Greece (from c. 460 BCE – beyond 500 BCE):
- Hippocrates (c. 460–370 BCE), the “Father of Medicine,” referred to TB as “phthisis” (consumption) and noted its high prevalence and often fatal nature, especially among young adults. He provided clinical descriptions of symptoms like chronic cough, chest pain, and wasting.
By 500 BCE, tuberculosis had become a well-established and widespread disease globally, leaving its indelible mark on early human populations and the emerging civilizations around the world.
TB Medieval and Early Modern Eras (500 CE – 1800 CE): Middle Ages
During the Middle Ages (roughly 500 CE to 1500 CE), Tuberculosis (TB) continued its pervasive presence in Europe, although the medical understanding of its cause and transmission remained limited. Often referred to as “consumption” or “phthisis” (from the ancient Greek for “wasting away”), TB was a chronic and often fatal disease that silently afflicted populations alongside more dramatic epidemics like the plague.
Prevalence and Impact in Medieval Society:
- Endemic Nature: Unlike the explosive outbreaks of plague, TB was an endemic disease. It was consistently present in communities, causing long-term illness and death, primarily due to pulmonary (lung) tuberculosis.
- Skeletal Evidence: Paleopathological studies of medieval skeletal remains frequently show evidence of TB, particularly in the spine (Pott’s disease), joints, and ribs. This confirms its widespread presence across various social strata. For example, studies in early medieval Switzerland and medieval Denmark have found lesions consistent with TB, suggesting a significant prevalence.
- The “King’s Evil” (Scrofula): A common manifestation of TB in the Middle Ages was scrofula, or TB of the lymphatic nodes, often in the neck. This condition caused visible swellings, and in England and France, it became known as the “King’s Evil” (mal du roi). It was widely believed that the touch of the monarch (the “royal touch”) could miraculously cure scrofula due to their divine right. This practice was immensely popular, with kings like Edward I of England reportedly touching thousands. While medically ineffective, it highlights the desperation for cures and the blend of spiritual and physical beliefs surrounding illness.
Understanding and “Treatments”:
- Lack of Germ Theory: Without any understanding of microorganisms, medieval medicine generally attributed diseases like TB to imbalances in the four humors (blood, phlegm, yellow bile, and black bile), astrological influences, or even divine punishment.
- Symptomatic Management: Treatments for “consumption” were largely palliative and aimed at rebalancing the humors or alleviating symptoms. These included:
- Dietary changes: Often recommending rich foods and milk.
- Herbal remedies: Various herbs were used for coughs and respiratory issues.
- Bloodletting and purging: Common practices to remove perceived excess humors.
- Rest: Basic recommendations for recovery.
- Contagion (Limited Recognition): Although the contagious nature of TB was occasionally speculated upon (e.g., by Avicenna in the Arabic Empire, whose medical texts influenced Europe), it was not consistently understood or acted upon. The idea of “miasma” (bad air) as a cause was more prevalent than person-to-person transmission.
- Sanatorium Precursors: The concept of fresh air and good diet, later central to the sanatorium movement, did exist in rudimentary forms. Some ancient physicians and later medieval practitioners might have recommended changes in climate or environment.
The Middle Ages were a period when TB was a consistent, low-level, but highly lethal killer. Its pervasive nature and the visible suffering it caused were deeply integrated into daily life and popular beliefs, even as its true biological mechanisms remained a mystery.
TB Medieval and Early Modern Eras (500 CE – 1800 CE): Growing Understanding of Contagion
During the long span of the Medieval and Early Modern Eras (500 CE – 1800 CE), the understanding of Tuberculosis (TB) gradually began to shift from purely humoral or divine explanations towards a nascent, though often incomplete, grasp of its contagious nature. This evolution was slow and fragmented, predating the germ theory by centuries, but it laid critical groundwork.
Early Glimmers of Contagion (Medieval Influences):
- Arabic Medical Scholars (9th-11th Centuries): While European medieval medicine was often rooted in humoral theory, influential Arabic physicians offered more advanced insights. Avicenna (Ibn Sina, c. 980-1037 CE), whose “Canon of Medicine” was a foundational text in both the Islamic world and later in Europe, was one of the earliest to suggest that tuberculosis might be contagious. He noted that consumption could be spread, though the mechanism remained unclear.
- Isolation Practices: In some medieval communities, particularly in monastic settings or areas with public health concerns, there were sporadic practices of isolating individuals with advanced TB, suggesting an intuitive, if unscientific, recognition that close contact was dangerous. However, these were not widespread or systematically applied.
The Renaissance and Early Modern Era (15th – 18th Centuries): As Europe emerged from the Middle Ages, observations became more systematic, though often still intertwined with older theories.
- Growing Suspicion (16th-17th Centuries): With increased population density in burgeoning cities, the prevalence of TB became undeniable. Physicians and lay observers alike began to notice patterns: families sharing rooms were more likely to contract it, and young people caring for the sick often fell ill themselves. This led to a stronger, albeit unproven, suspicion of contagion.
- Fracastoro’s Theory of Contagion (1546): Italian physician Girolamo Fracastoro published De Contagione et Contagiosis Morbis (On Contagion and Contagious Diseases). He proposed that diseases were caused by minute, unseeable, self-propagating “seeds” or “seminaria” that could be transmitted by direct contact, fomites (contaminated objects), or even from a distance. While not specific to bacteria or viruses, his general theory of contagious seeds was a significant intellectual leap that supported the idea of TB’s transmissibility.
- Legal Measures in Southern Europe (17th-18th Centuries): Some of the earliest public health measures based on a suspected contagious nature of TB appeared in Southern Europe, particularly in Italy and Spain. These regions had experienced devastating plagues and were more attuned to contagion theories.
- Naples and Tuscany (17th-18th Centuries): Decrees were issued requiring the reporting of TB cases and the disinfection or destruction of belongings (bedding, clothing) of those who died from consumption. This was a direct, albeit harsh, response to the belief that the disease could be transmitted from the sick person or their environment. These measures were controversial and often difficult to enforce.
- Morgagni’s Observations (18th Century): The Italian anatomist Giovanni Battista Morgagni (1682-1771), considered the father of modern anatomical pathology, provided detailed descriptions of tubercular lesions found during autopsies. His work contributed to a more precise understanding of the physical progression of the disease, indirectly supporting the idea of a specific agent.
- Limited Acceptance: Despite these observations and nascent theories, the dominant medical paradigm remained a humoral theory. The idea that invisible particles could cause disease was radical, and the specific mechanism of TB’s spread (airborne droplets) was not truly understood. Many still believed it was hereditary, caused by poor diet, or a result of an individual’s constitution.
While lacking the scientific rigor of later discoveries, the Medieval and Early Modern Eras saw a slow but discernible shift toward recognizing TB as a transmissible disease. These early intuitions and sporadic public health measures, though imperfect, set the stage for the crucial scientific breakthroughs of the 19th century.
TB Medieval and Early Modern Eras (500 CE – 1800 CE): “The White Plague” / Consumption (17th – 18th Centuries)
During the 17th and 18th centuries, tuberculosis (TB) reached epidemic proportions in Europe and North America, earning grim nicknames like “The White Plague” and universally known as “Consumption.” This era represents a peak in the disease’s devastating impact before the advent of scientific understanding and effective treatments.
“Consumption”: The Wasting Disease The most common and enduring name for TB during this period was “consumption.” This term graphically describes the observable progression of the disease:
- Wasting Away: Sufferers would experience a gradual, relentless wasting of their bodies, losing significant weight, muscle mass, and vitality. It appeared as though the disease was literally “consuming” them from within, leading to severe emaciation.
- Symptoms: This “consuming” process was accompanied by a chronic, often hacking cough (frequently producing blood-tinged sputum), fever, night sweats, and profound weakness. The slow, degenerative course of the illness, culminating in a spike in fever and often choking on blood, made for a terrible and prolonged demise.
“The White Plague”: A Reflection of Appearance and Prevalence The term “The White Plague” emerged in the 18th century to describe tuberculosis, primarily due to two key reasons:
- Pale Complexion: Patients often developed an extreme anemic pallor, giving their skin a ghostly white appearance, contrasted by the flushed cheeks that fever could induce. This “consumptive aesthetic” of delicate thinness and pale skin even became, paradoxically, a fashionable ideal in some circles, romanticized in art and literature, particularly for women.
- Widespread Devastation: “Plague” signifies a widespread and deadly epidemic. While not as sudden and dramatic as the bubonic plague, TB was consistently prevalent and caused immense mortality. In the 18th century, it was the single leading cause of death in many European cities and in colonial America.
- Estimates suggest TB was responsible for as much as one-quarter of all deaths in Europe and the United States during parts of the 18th and 19th centuries. For instance, in 1815, one in four deaths in England was due to consumption.
- In densely populated urban centers like London, around 1% of the population could die from TB annually by the mid-18th century.
Societal Factors Fueling the Epidemic: The rise of TB during this era was intrinsically linked to profound societal changes:
- Industrial Revolution: Rapid urbanization, driven by the Industrial Revolution, led to unprecedented overcrowding in cities. Poorly ventilated housing, unsanitary conditions, and new forms of factory and workshop labor created ideal environments for the airborne spread of Mycobacterium tuberculosis.
- Malnutrition and Poverty: The widespread poverty and malnutrition common among the burgeoning urban working classes weakened their immune systems, making individuals more susceptible to developing active disease if infected. While TB affected all social classes, it disproportionately impacted the poor.
- Lack of Understanding: Despite growing suspicions of contagion, the true bacterial cause of TB was not discovered until 1882 by Robert Koch. Without this understanding, effective prevention strategies were absent, and treatments remained largely ineffective, contributing to its relentless spread and death toll.
“The White Plague” was a pervasive and terrifying reality of life in the 17th and 18th centuries, a silent but constant killer that shaped demographics, social structures, and cultural perceptions of illness and death.
TB The Industrial Age and Scientific Breakthroughs (19th Century)
The 19th century was a paradox for tuberculosis (TB): it was the era when the disease reached its most devastating peak, fueled by the Industrial Revolution, yet it also witnessed the revolutionary scientific breakthroughs that would fundamentally transform its understanding and eventually lead to its decline.
The Industrial Age: Fuelling the Epidemic: The rapid industrialization of the 19th century created conditions that allowed TB, often still called “Consumption” or “The White Plague,” to flourish and claim more lives than ever before.
- Urban Overcrowding: Millions migrated from rural areas to burgeoning cities in search of work, leading to unprecedented population density. People were packed into small, poorly ventilated tenements.
- Deplorable Living Conditions: Sanitation was often abysmal, and many lived in squalor, lacking adequate nutrition and sunlight.
- Hazardous Working Environments: Factories, mines, and workshops were frequently damp, dusty, and poorly ventilated, where workers spent long hours in close proximity, ideal for the airborne transmission of Mycobacterium tuberculosis.
- Social Impact: TB became the leading cause of death in Western Europe and North America, killing one in four adults in many major cities. It disproportionately affected the urban poor but also claimed prominent figures in art, literature, and music (e.g., the Brontë sisters, John Keats, Frédéric Chopin). Ironically, the lingering pallor and ethereal thinness associated with the disease were sometimes romanticized in art and fashion.
Pivotal Scientific Breakthroughs: Despite the widespread devastation, the 19th century laid the groundwork for modern understanding and control of TB through critical scientific discoveries:
- Jean-Antoine Villemin Proves Contagion (1865):
- While suspicion of TB’s contagious nature existed, it was French military surgeon Jean-Antoine Villemin who definitively proved it. In 1865, he conducted groundbreaking experiments, demonstrating that tuberculosis could be transmitted by inoculating rabbits with tuberculous material from human cadavers. This conclusively showed that TB was an infectious disease caused by a specific transmissible agent, not a hereditary condition or simply due to lifestyle.
- Robert Koch Discovers the Tubercle Bacillus (1882):
- This was the most monumental breakthrough. On March 24, 1882, German physician and microbiologist Robert Koch announced to the Berlin Physiological Society that he had identified the bacterium responsible for tuberculosis: Mycobacterium tuberculosis (the “tubercle bacillus”).
- Koch meticulously isolated, cultured, and then used this pure culture to induce TB in animals, fulfilling his famous “Koch’s Postulates” and definitively proving the bacillus was the cause of the disease. This discovery, for which he later received the Nobel Prize, transformed TB from a mysterious, often romanticized illness into a tangible, identifiable public health problem. March 24 is now celebrated as World TB Day.
- René Laennec and the Stethoscope (1816):
- While not discovering the germ itself, French physician René Laennec invented the stethoscope in 1816. This revolutionary diagnostic tool allowed doctors to listen to internal body sounds, particularly in the lungs. Laennec used it to meticulously correlate specific sounds with the characteristic lesions of pulmonary TB seen during autopsies. His work, detailed in his 1819 treatise, laid the foundation for clinical diagnosis of the disease.
- Wilhelm Röntgen and X-rays (1895):
- Towards the end of the century, German physicist Wilhelm Röntgen’s discovery of X-rays in 1895 provided another powerful diagnostic tool. X-rays allowed physicians to visualize the lesions and damage caused by TB in the lungs of living patients, offering a non-invasive way to diagnose and monitor the disease.
Early Public Health Responses: Armed with the knowledge of contagion, public health efforts began to shift:
- Sanatorium Movement: Building on earlier concepts of fresh air and rest, the sanatorium movement gained significant traction. The first modern sanatorium was opened by Hermann Brehmer in Silesia in 1854. These facilities aimed to isolate infectious patients to prevent spread and provide a regimen of rest, nutritious food, and fresh air, believed to boost recovery (though not a cure).
- Public Awareness Campaigns: Understanding that TB was contagious led to early public health campaigns advising hygiene measures like spitting into receptacles, covering coughs, and improving ventilation. However, this also, unfortunately, led to increased stigma and social ostracism for TB sufferers.
The 19th century was a turning point for TB. The disease’s devastating presence highlighted the urgent need for solutions, and the remarkable scientific discoveries laid the essential groundwork for the prevention and treatment strategies that would emerge in the 20th century.
TB The Modern Era: Treatment, Decline, and Resurgence (20th Century – Present)
The 20th century opened with tuberculosis (TB) as the leading cause of death globally, but it also witnessed a revolutionary transformation in TB control with the advent of antibiotics. However, this success was later challenged by new global health crises and the emergence of drug-resistant strains, leading to a complex modern era of decline and resurgence.
The Antibiotic Revolution and the “Magic Bullet” (Mid-20th Century): The true turning point in the fight against TB came with the discovery of effective antimicrobial drugs.
- Streptomycin (1943): Discovered by Albert Schatz in Selman Waksman’s lab, streptomycin was the first effective antibiotic against Mycobacterium tuberculosis. Its introduction in the mid-1940s marked the beginning of chemotherapy for TB.
- Combination Therapy: Soon, other powerful drugs followed, including para-aminosalicylic acid (PAS) in 1945 and isoniazid (INH) in 1952. A crucial realization was that using a combination of multiple drugs (e.g., streptomycin, INH, PAS) was essential to prevent the rapid development of drug resistance and to achieve a cure. This multi-drug regimen dramatically reduced treatment times from years in sanatoria to months.
- Rapid Decline in Developed Nations: The availability of these drugs, combined with improved living conditions, nutrition, and public health interventions (like mass X-ray screenings), led to a dramatic and rapid decline in TB incidence and mortality in developed countries from the 1950s onwards. Many believed TB would soon be eradicated.
Vaccine Development (20th Century):
- BCG Vaccine (1921): The Bacillus Calmette-Guérin (BCG) vaccine, developed by Albert Calmette and Camille Guérin, was first used in humans in 1921. While not fully effective against adult pulmonary TB, it has been widely used, particularly in high-burden countries, to protect infants and young children from severe forms of the disease.
The Resurgence and New Challenges (Late 20th Century – Present): The optimism of eradication faded in the late 20th century as TB experienced a global resurgence, particularly after the 1980s.
- HIV/AIDS Pandemic: This was the single most significant factor in TB’s resurgence. The Human Immunodeficiency Virus (HIV) severely weakens the immune system, making individuals far more susceptible to developing active TB disease if they are infected with the bacteria (which is common globally). TB became the leading cause of death among people living with HIV/AIDS.
- Drug Resistance: Inadequate treatment regimens (patients not completing their full course of drugs), improper prescribing, and poor drug supply led to the evolution of drug-resistant strains of TB.
- Multidrug-Resistant TB (MDR-TB): Resistant to at least the two most powerful first-line anti-TB drugs (isoniazid and rifampicin).
- Extensively Drug-Resistant TB (XDR-TB): Resistant to isoniazid and rifampicin, plus any fluoroquinolone and at least one of the three injectable second-line drugs.
- These drug-resistant forms are much harder, more expensive, and longer to treat, with lower cure rates.
- Weakened Health Systems: In many parts of the world, particularly in countries with high TB burdens, health systems struggle with funding, infrastructure, and trained personnel, making it difficult to implement effective TB control programs.
- Poverty and Social Determinants: Poverty, malnutrition, overcrowding, and inadequate ventilation remain major drivers of TB, especially in marginalized communities and urban slums.
Global Efforts and Current Status (21st Century):
- DOTS Strategy: In the 1990s, the WHO introduced the Directly Observed Treatment, Short-course (DOTS) strategy, emphasizing supervised drug intake to ensure adherence and prevent resistance. This later evolved into the broader “Stop TB Strategy.”
- Newer Drugs and Diagnostics: The 21st century has seen the development of some new anti-TB drugs (e.g., bedaquiline, delamanid) and faster, more accurate diagnostic tools (e.g., GeneXpert), offering new hope against drug-resistant forms.
- Persistent Global Burden: Despite significant efforts, TB remains a major global health challenge. According to the WHO, it is still one of the top infectious killers worldwide, responsible for over a million deaths annually. While the incidence rate has been slowly declining globally, the fight against drug resistance and achieving equitable access to diagnosis and treatment remain critical priorities for the present and future.
Bubonic Plague, Smallpox, and Tuberculosis (TB): Similarities
The Bubonic Plague, Smallpox, and Tuberculosis are all infectious diseases that have had profound impacts on human history. While caused by different pathogens and with distinct clinical presentations, they share several crucial similarities:
- Infectious Agents: All three are caused by specific infectious agents:
- Bubonic Plague: The bacterium Yersinia pestis.
- Smallpox: The variola virus.
- Tuberculosis: The bacterium Mycobacterium tuberculosis.
- Historical Epidemic/Pandemic Impact: Each disease has caused widespread suffering and massive loss of life throughout history, earning them grim reputations:
- Bubonic Plague: Responsible for the “Black Death” and other devastating pandemics that depopulated vast regions.
- Smallpox: Historically, one of the greatest killers, leading to millions of deaths and widespread disfigurement over centuries.
- Tuberculosis: Often called “the white plague” or “consumption,” it has been a chronic and pervasive killer for millennia, particularly during periods of urbanization and poverty, and remains a major global health problem.
- Spread through Human Populations: All three diseases are transmissible among humans, leading to their ability to spread widely:
- Bubonic Plague: While primarily zoonotic (from animals via fleas), human-to-human transmission occurs with the pneumonic form.
- Smallpox: Primarily spread person-to-person via respiratory droplets and direct contact.
- Tuberculosis: Primarily spreads from person to person via airborne droplets from infected individuals.
- Significant Societal and Economic Impact: Beyond mortality, these diseases profoundly affected human societies:
- Demographic Shifts: Caused significant population declines, altering labor forces and social structures.
- Economic Disruption: Disrupted trade, agriculture, and urban life.
- Fear and Stigma: Generated immense fear, leading to isolation of the sick and social stigma.
- Medical Advancements: Drove significant advancements in medical understanding, public health, and immunology (e.g., the development of the first vaccine for smallpox and later, antibiotics for plague and TB).
- Targeted by Public Health Measures: Historically and currently, these diseases have been (or were) targets of intense public health interventions:
- Quarantine: Used for all three, especially during severe outbreaks, to limit the spread.
- Sanitation/Hygiene: While differing in direct impact, general improvements in living conditions (less crowding, better ventilation) helped reduce the spread of all three.
- Surveillance: Tracking cases and contacts to monitor and control outbreaks.
- Potential for Severe Outcomes/Mortality (especially untreated): Without effective intervention or treatment, all three diseases can be deadly:
- Bubonic Plague: Untreated, has a very high mortality rate (50-90%).
- Smallpox: Variola major had a mortality rate of up to 30% or more.
- Tuberculosis: Untreated active TB has a high mortality rate, particularly in immunocompromised individuals (e.g., people living with HIV).
- Immunological Response: The human immune system attempts to fight off all three infections, though with varying degrees of success and different types of immune responses. This understanding was critical for vaccine development for smallpox and diagnostic tests for TB.
In summary, while distinct in their microbiology and disease progression, Bubonic Plague, Smallpox, and Tuberculosis stand as three of the most impactful infectious diseases in human history, sharing a common legacy of widespread suffering, societal disruption, and driving forces behind medical and public health progress.
Bubonic Plague, Smallpox, and Tuberculosis (TB): Differences
While Bubonic Plague, Smallpox, and Tuberculosis share some broad similarities as historical infectious diseases, their differences are fundamental and crucial for understanding their impact and how they were (or are) controlled.
Here’s a breakdown of their key differences:
- Causative Agent
- Bubonic Plague: Caused by a bacterium, Yersinia pestis.
- Smallpox: Caused by a virus, the variola virus (VARV).
- Tuberculosis (TB): Caused by a bacterium, Mycobacterium tuberculosis.
This distinction is fundamental because it dictates treatment options. Bacteria respond to antibiotics, while viruses do not (though antivirals exist for some, they work differently).
- Primary Mode of Transmission
- Bubonic Plague: Primarily a zoonotic disease. It’s usually transmitted to humans through the bite of infected fleas that have fed on infected rodents (like rats). Human-to-human transmission is rare for bubonic plague itself, but the more severe pneumonic plague (when the infection reaches the lungs) can spread person-to-person via respiratory droplets.
- Smallpox: Exclusively a human-to-human disease. It spreads directly from person-to-person through large respiratory droplets from close, face-to-face contact, or, less commonly, through contaminated materials (fomites). There was no animal reservoir.
- Tuberculosis (TB): Primarily an airborne disease. It spreads from person-to-person when an individual with active pulmonary TB coughs, sneezes, or speaks, releasing tiny airborne particles containing the bacteria that others can inhale.
- Clinical Presentation & Symptoms
- Bubonic Plague: Characterized by the sudden onset of fever, chills, and the hallmark buboes – severely swollen, painful lymph nodes, usually in the groin, armpit, or neck. If untreated, it can progress rapidly to septicemic (bloodstream infection) or pneumonic (lung infection) forms, often leading to rapid death.
- Smallpox: Began with a high fever, body aches, and malaise, followed by a distinctive rash that progressed through stages (macules, papules, vesicles, pustules, scabs) appearing synchronously over the entire body, leading to characteristic pitted scars (pockmarks).
- Tuberculosis (TB): Often a chronic disease, particularly affecting the lungs. Symptoms develop gradually over weeks or months and include a persistent cough (sometimes with blood), fever, night sweats, weight loss (“consumption”), and fatigue. It can also affect other organs (extrapulmonary TB). It has a latent phase where individuals are infected but asymptomatic and non-contagious.
- Disease Progression and Latency
- Bubonic Plague: Typically acute and rapidly progressive. Without treatment, it often leads to death within days. No prolonged latent stage in humans for the bubonic form.
- Smallpox: Acute, with a defined incubation period and disease course. Survivors were immune, but there was no long-term latent infection that could reactivate.
- Tuberculosis (TB): Characterized by a significant latent phase. A majority of infected individuals (90-95%) do not develop active disease but harbor the bacteria in a dormant state for years or decades. This latent infection can reactivate later, especially if the immune system weakens.
- Preventative Measures & Eradication Status
- Bubonic Plague:
- Prevention: Control of rodent populations and fleas, avoidance of infected animals, rapid treatment with antibiotics for contacts.
- Eradication Status: Not eradicated. It still exists in natural foci around the world (enzootic cycles) and causes sporadic human cases and localized outbreaks.
- Vaccine: A vaccine exists but is generally reserved for high-risk individuals (e.g., laboratory workers, military personnel in endemic areas).
- Smallpox:
- Prevention: Vaccination was the key. Edward Jenner’s cowpox-based vaccine provided effective, long-lasting immunity.
- Eradication Status: Officially eradicated globally in 1980. This was possible because it only infected humans (no animal reservoir), the vaccine was highly effective, and visible symptoms allowed for effective “ring vaccination” (surveillance and containment).
- Vaccine: The reason for its eradication.
- Tuberculosis (TB):
- Prevention: BCG vaccine (variable effectiveness, mainly in children against severe forms), early diagnosis and treatment of active cases, and treatment of latent TB infection.
- Eradication Status: Not eradicated. It remains a major global public health problem, exacerbated by drug resistance and co-infection with HIV.
- Vaccine: BCG vaccine, but new, more effective vaccines are desperately needed.
In essence, while all three were historical scourges, smallpox’s unique biology (human-only host) made it eradicable with an effective vaccine. Plague’s zoonotic nature means it persists in animal populations. TB’s unique ability to enter a latent phase and its airborne spread in a chronic form make it incredibly difficult to eliminate, especially with the added challenge of drug resistance and its strong links to poverty.
Bubonic Plague, Smallpox, and Tuberculosis (TB) Compared: Epidemics and Diseases
The Bubonic Plague, Smallpox, and Tuberculosis (TB) are three of the most impactful diseases in human history, each with distinct characteristics, modes of transmission, and historical trajectories.
Here’s a comparison of these three formidable epidemics and diseases:
| Feature | Bubonic Plague | Smallpox | Tuberculosis (TB) |
| Pathogen Type | Bacterium (Yersinia pestis) | Virus (Variola virus) | Bacterium (Mycobacterium tuberculosis) |
| Primary Transmission | Flea bites (from infected rodents); direct contact with infected tissues/fluids; respiratory droplets (pneumonic form, person-to-person). | Prolonged face-to-face contact (respiratory droplets); direct contact with skin lesions or contaminated items (fomites). | Airborne droplets (when an infected person coughs, sneezes, or speaks). |
| Reservoir(s) | Rodents (e.g., rats, squirrels) and their fleas | Humans only (no animal reservoir) | Humans primarily, occasionally animals (e.g., cattle for bovine TB). |
| Key Symptoms | Sudden fever, chills, headache, weakness, painful swollen lymph nodes (buboes). It can progress to septicemic (blood infection) or pneumonic (lung infection). | High fever, body aches, followed by a distinctive rash that progresses to fluid-filled pustules, which scab over and leave scars. | Persistent cough (often accompanied by blood), chest pain, fever, night sweats, weight loss, and fatigue. (Can affect lungs or other organs). |
| Historical Mortality | Extremely High. The Black Death (14th century) killed 30-60% of Europe. Total deaths across pandemics: hundreds of millions. | Very High. Killed ~3 in 10 infected. An estimated 300 million in the 20th century alone before eradication. | Very High. Known as “consumption” or “white plague.” Historically, it has been a leading cause of death for centuries. |
| Modern Status | Rare; treatable with antibiotics if diagnosed early. Endemic in parts of Africa (Madagascar, DRC), Peru, and some rural areas globally. | Globally Eradicated in 1980. No naturally occurring cases exist. | Remains a top infectious killer worldwide (1.5 million deaths/year). Curable with a long course of antibiotics, but drug-resistant strains are a major threat. |
| Prevention/Control | Rodent and flea control, improved sanitation, and antibiotics for treatment and post-exposure prophylaxis. | Vaccination (key to eradication); isolation of cases; surveillance and ring vaccination. | Improved living conditions and nutrition; vaccination (BCG, although with variable effectiveness); early detection and adherence to a full antibiotic treatment course. |
Export to Sheets
Each of these diseases has left an indelible mark on human history, driving significant public health advancements and societal changes. Smallpox stands as a unique triumph of global health, while plague and TB continue to pose challenges, though their impact is vastly diminished in areas with modern medical care.



